This is the first of two interesting wound healing studies done in nude mice implanted with different biological material. Full thickness skin wounds were created on the dorsum and then different dermal replacements were implanted. Healing was followed for 28 days and very detailed histological and immunostaining evaluations of the wounds were performed to quantify the healing process. Most studies of wound healing use simple or highly subjective measures of healing, but one of the main goals of these studies was to use objective criteria to quantify healing and to do so in fine detail.
Healing and Neovascularization of Wounds Implanted with Dermal Substitutes
Healing and
Neovascularization of Wounds
Implanted
with Dermal Substitutes and Fibrin Glue
in Nude
Mice
Performed
at the Sumner L. Koch Burn Center, Department of Trauma
John
Stroger Jr. Hospital of Cook
County 1 &
Department
of General Surgery 2
Rush University Medical Center,
Chicago, Illinois
Michele M . Loor, MD
Burn Research Fellow
(2003-2005)
Department of General
Surgery
Rush University
Medical Center
Anh-Tuan Truong, MD
Burn Research Fellow
(2001-2002)
Metropolitan Group Hospitals
Residency in Surgery Program
Barbara A. Latenser, MD 1,2,3
Director, Burn Services
Dorion E. Wiley, MD 1,2
Attending Physician,
Department of Trauma, Burn Unit
Robert J. Walter, PhD 1,2
Senior Scientist
Department of Trauma
Address Correspondence to:
Robert
J. Walter, PhD
Department
of Trauma, Suite 1300
John
Stroger Jr. Hospital of Cook
County
1900 West Polk Street
Chicago, IL
60612
Phone: 312.864.0578
Email: rwalter@rush.edu
3
Current
address: University
of Iowa Hospitals and Clinics, Department of
Surgery, Section of Trauma, Burn, and Critical
Care, Iowa City, IA
ABSTRACT
Background: Dermal substitutes implanted into
full-thickness skin wounds reduce wound contraction and improve cosmesis. These improvements depend upon the
development of optimal dermal vascularization.
Methods: Full-thickness skin wounds were created on
the dorsum of nude mice. A dermal matrix
was implanted followed by a mix of fibrin glue (FG) with human keratinocytes
(KC). The following dermal matrices were
used: Integra, AlloDerm, ADM, Dermalogen, and Dermagraft. Wound healing was observed for 4 weeks. Biopsies were immunostaining for laminin
followed by blood vessel quantitation in the superficial and deep dermis in
three regions: wound center, wound
margin, and unwounded dermis.
Results: Extensive vascularity was
seen at all time points in implanted Dermagraft and Dermalogen. AlloDerm showed limited vascularity within
the first 2 weeks but this normalized by day 28. ADM and Integra showed rapid but controlled
ingrowth of vessels from both the wound base and margins.
Conclusions: Dermagraft underwent extensive granulation
whereas AlloDerm and Dermalogen underwent delayed vascularization. AlloDerm, Integra, and ADM underwent
progressive vessel ingrowth that seemed to be conducive to normal dermal
regeneration and modest wound contraction.
Key
words: neovascularization, angiogenesis,
wound healing, dermal substitute, nude mouse, Integra, AlloDerm, ADM,
Dermagraft, Dermalogen, ACIS
INTRODUCTION
The treatment of full-thickness wounds in surgery,
trauma, and burn poses a significant clinical challenge for several
reasons. An open wound not only provides
a portal of entry for microorganisms to invade and proliferate, but it also
allows vital fluid and electrolytes to escape. The primary objective in
treating an open wound is, therefore, early coverage. A secondary consideration is optimizing the
function and appearance of the healed wound.
Since full-thickness wounds lack a dermis and the dermis does not
regenerate, they tend to heal slowly and with significant scarring. Various
synthetic collagen-based dermal matrices are now available for use in wounds.1-11 Dermal replacements are intended to provide
rapid wound coverage and improve wound healing.
Once implanted, these replacements either become incorporated into the
wound or stimulate tissue growth. However, the efficacy of these materials in
the treatment of full-thickness wounds has not been studied carefully and
compared.
In most cases, placement of a dermal matrix is later
followed by additional surgery for split-thickness skin grafting in order to
achieve definitive wound closure.
The use of
cultured autologous keratinocytes has been studied extensively as an
alternative to autografting, particularly in patients with greater than 40%
total body surface area wounds who have limited donor site availability.12-19
Various methods of keratinocyte (KC) delivery to wounds are available including
combination with a fibrin sealant. Several
studies have suggested the utility of fibrin glue (FG) in this setting, with
evidence to suggest enhanced reepithelialization and basement membrane
formation.20-24 Cultured KCs can also be introduced into wounds via
a spray apparatus.25
Preliminary studies in which KCs were suspended in fibrin glue and
sprayed onto wounds indicate that this permits acceptable cell survival and
proliferation.26-29 Other potential advantages of the use of fibrin
glue in wounds are improved hemostasis and protection from infection.30
A KC spray apparatus has been developed in our lab for use following suspension
of the KCs in the thrombin component of fibrin sealant (Tisseel®; Baxter,
Deerfield, IL). It has tested both in vitro and in vivo, and the viability and proliferative potential of the KCs
immediately and 24-48 hours after spraying are affected very little.
Neovascularization is a key step in wound healing,
as new vessels are necessary to support the newly formed tissue. It is a complex process that is dependent
upon appropriate interactions between the extracellular matrix, the migrating
endothelial cells, and a number of growth factors.31 Dermal substitutes composed of native
(undenatured), allogenic extracellular matrix such as acellular dermal matrix
(ADM) and AlloDerm become readily vascularized, complement healing, and reduce
contractive scarring in a variety of wound types. We hypothesized that the mode and rate of
neovascularization during the process
of wound healing in the presence of ADM or AlloDerm may be an important
determinant in final wound resolution.
The process of vascularization may be significantly altered with the use
of other materials such as those containing synthetic, xenogenic, or denatured
substances including Integra, Dermalogen, and Dermagraft, thereby negatively
affecting wound resolution.
To evaluate this, dermal substitutes in conjunction
with FG and KC were introduced into full-thickness wounds on the dorsum of nude
mice. The progress of wound healing and
particularly dermal neovascularization was evaluated by objective criteria over
a period of four weeks.
Paraffin-embedded sections from weekly biopsies were immunostained for
laminin, an antigen found in the endothelial basement membrane, to assess and
compare vascularization in these healing wounds.
METHODS AND
MATERIALS
The following materials were used:
§
Tisseel® (Baxter Health, Deerfield,
IL) is a two component system in
which fibrinogen, calcium, thrombin, and a protease inhibitor are combined and
dispensed onto a wound or other surface to form a fibrin clot.
§
Integra® (Ethicon, Somerville, NJ) is a bilayer artificial skin
composed of a “dermal” layer of bovine collagen gel cross-linked with shark
chondroitin-6-sulfate and an “epidermal” layer of polysiloxane polymer (which
was removed for this study).
§
AlloDerm® (Lifecell Corp., Branchburg,
NJ) is a collagen matrix derived
from human skin that is treated to remove most of the cellular components.
§
Acellular Dermal Matrix (ADM) is
a dermal collagen matrix derived from human skin that is treated to remove all
cellular components.11, 31Dermalogen® (Collagenesis Corp, Beverly, MA)
is a powdered human dermal matrix that has been treated to remove some cellular
components and contains collagens, fibronectin, and elastin. This matrix material is supplied as a 3.5%
suspension in phosphate buffer.
§
Dermagraft® (Advanced Tissue Sciences) is comprised of a woven
bioabsorbable polymer on and in which human dermal fibroblasts are grown and
then devitalized.
Animals and Surgery
NIH homozygous male nude mice, 4 weeks of age
(Taconic, Germantown, NY) were used. This model permits the implantation of
xenogenic materials, such as those present in these dermal substitutes (e.g.,
bovine collagen, shark chondroitin sulfate, human collagens, human fibroblasts
and KCs). The disadvantage of this model
is that the effect on neovascularization of immune reactivity against such
xenogenic materials that might be evinced in humans will be masked or absent.
All surgical interventions and experiments were
performed in the John H. Stroger, Jr. Hospital of Cook County Animal Care
Facility using protocols approved by the IACUC.
Preoperative antibiotics (Kanamycin 25U/kg IM x 1 dose) were
administered to the animals and ketamine/ xylazine was used for
anesthesia. Under aseptic conditions, 2
cm x 2 cm full-thickness wounds were excised down to the muscle fascia,
removing the panniculus carnosus. The
groups used in this experiment were as follows: 1) Integra, 2) AlloDerm, 3)
ADM, 4) Dermalogen, 5) Dermagraft, 6) KCs + FG only, and 7) FG only. Animals in groups 1-6 received human KCs
sprayed onto the wound surface in combination with FG. Each group was comprised of at least 6 mice,
three of which underwent biopsies at days 7, 14, 21 and 28. Three more mice were treated as in groups 1-6
except that FG was administered without KCs.
In the groups of mice that received dermal substitutes, the substitute
was cut to size and sutured to place in the wound using 4-0 nylon sutures. In the Dermalogen group, 2 cc of a suspension
was placed in the wound. In the groups
of mice to receive KCs, KCs at a concentration of 104 per cc in
Tisseel (0.5 cc) were sprayed on the dermal matrix. All defects were covered with a
semi-permeable adhesive film (Op-Site, Smith & Nephew, Largo, FL), Xeroform
(Sherwood Medical, St. Louis, MO), dry cotton gauze (Adaptic, Johnson &
Johnson, New Brunswick, NJ), and finally with a fine stainless steel mesh fixed
to the animals’ back with skin sutures.
This last was used to prevent the wounds from being disturbed by chewing
or scratching. Dressings were inspected
daily. At the completion of the study
period (4 weeks), all animals were euthanized.
The harvested biopsies were fixed in 10% buffered formalin. Specimens were paraffin embedded and sections
stained with H&E or immunostained.
Preparation of ADM
Thawed cadaver skin was treated with 2.5 units/ml
Dispase II (Boehringer Mannheim, Indianapolis,
IN) in PBS containing 0.2 mM CaCl2
at 4EC for 24 hours to remove the
epidermis and other cellular components from the dermal matrix. Subsequently, the dermal matrix was incubated
in buffered 0.5% Triton X-100 (U. S. Biochemical Corp., Cleveland, OH) for 24
hours at room temperature with continuous shaking. ADM was then extensively washed with PBS and
stored in PBS at 4EC until use 11,31.
Human KC Culture and Preparation for Spraying
Human KCs, Epilife culture medium, supplements, and
transfer solutions (trypsin, trypsin neutralizer) were obtained from Cascade
Biologics (Portland, OR).
KCs arrived tested and warranted to be free of HIV, hepatitis B and C,
mycoplasma, bacteria, yeast and other fungi.
Cells were grown in 75 cm2 flasks from expanded frozen stocks
stored after passage 2. After 7-10 days
of proliferation and growth, flasks containing 50-80% confluent KCs were
washed, trypsinized briefly to release cells from the substrate, trypsin
neutralized, and the suspension centrifuged at 20xg for 5 min and 4ºC. The supernatant was discarded and the cells
resuspended in fresh Epilife medium. Cells were mixed with the reconstituted
thrombin/calcium component of the fibrin sealant kit at a 1:1 dilution. The fibrinogen component was also
reconstituted and diluted 1:1 with Epilife.
Both components were stored at 4ºC until sprayed onto wounds in mice as
indicated above. Viability of the cells
following mixing with the thrombin component of Tisseel and after spraying
using spray apparatus was confirmed using trypan blue staining.
Immunostaining
Paraffin sections were deparaffinized and then
antigen retrieval was performed by incubating specimens in pepsin (1mg/ ml, in
0.01N HCl) at 37ºC for 2 hours.
Following antigen retrieval, blocking of nonspecific binding was
accomplished by incubation in a solution of 1% bovine serum albumin in Tris
buffered saline, pH 7.9 for 20 min.
Sections were then incubated in rabbit anti-mouse laminin IgG (Sigma,
St. Louis, MO) at a 1:10 dilution for 2 hr at room temperature followed by
HRP-conjugated goat anti-rabbit IgG secondary antibody (Cappel, Irvine, CA) at
a dilution of 1:100 for 1 hr at room temperature. Reaction product was generated using a Vector
DAB/peroxide Developing Kit (Vector, Burlingame,
VT) according to the
manufacturer’s specifications with a 10 min developing time. Specimens were counterstained with Vector
Hematoxylin QS (Vector, Burlingame,
VT) for one minute. Normal skin from previously unwounded mice
was also immunostained for laminin as above to serve as a control. Laminin staining identified the basement
membrane of epidermis, nerves, muscle, and vessels. Attention was focused on the dermis, within
which stained structures with a clear lumen were counted as blood vessels. These were readily distinguished from the
other positively stained structures by their location and shape. Immunostaining for endoglin (CD105) and CD34
was performed but the stain resulting was either nonspecific or too weak to be
of use here.
Data Analysis
Wound characteristics were measured grossly and
histologically. Gross observations were
made at days 7, 14, 21, and 28 post-surgery.
Wound contraction and degree of epithelialization were measured using
UTHSCSA ImageTool software in conjunction with digital photographs of wounds. Histological characteristics determined by
H&E staining included: the presence and thickness of a stratified
epithelial layer, the persistence of the implanted dermal matrix, and the
degree of inflammation. The degree of
vascularity was determined by two methods.
Manual vessel counts were carried out on the sections using an eyepiece
reticle. The size of this reticle grid
at the final magnification used (400X) was 250 x 250 μm. Numbers of blood vessels were determined by
counting vessels in the superficial dermis, i.e., with one side of the reticle
on the epidermal basement membrane and the counts performed in a 250 μm square
of dermis underlying the basement membrane or in the deep dermis, i.e., with
one side of the reticle positioned on the hypodermis and the counts performed
in 250 μm square of dermis directly overlying the hypodermis.
Three different zones of each wound biopsy were
evaluated in this way: the wound center (WC), wound margin (WM) and unwounded
normal dermis peripheral to the wound margin (NP). Slides were scanned visually at 40X to find
wholly intact sections showing all 3 regions of interest (WM, WC, and NP) and
then viewed at 400X magnification for vessel counts. Within each zone, two or more immediately
adjacent regions were counted and averaged.
Placement of the reticle was standardized by defining: the WC as the
region equidistant from each wound margin; the WM as the area directly adjacent
to unwounded tissue; and the NP as the area with normal skin structure and
appendages at least 500 µm away from the WM.
Additionally, some vessel counts were performed on laminin immunostained
tissue sections using the ChromaVision (San
Juan Capistrano, CA)
Automated Cellular Imaging System (ACIS) in conjunction with microvascular
density (MVD) software. Image analysis
was performed in each of these zones with one set of six counts obtained for
each region from which the microvascular density (#vessels/ mm2) was
calculated. Wounds that demonstrated
excessive blood vessel proliferation (more than 3 times the level seen in
unwounded mouse skin) were considered granulation tissue. Data from days 14 and 28 were analyzed with
one-way ANOVA and Tukey post-tests.
RESULTS
Gross wound observations
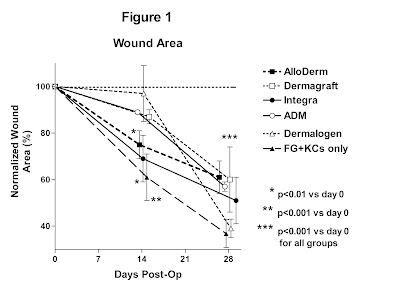
Each of the dermal substitutes
except Dermalogen reduced the amount of wound contraction as compared to wounds
that received no dermal substitute and sprayed KCs + FG (figure 1). Thirty-five to 45% wound contraction was
observed in wounds implanted with AlloDerm, Dermagraft, Integra, or ADM at 28
days post-operatively. Greater
contraction (60%) was observed in the Dermalogen and KCs + FG groups (figures
2, 3).
H&E staining
Histologically,
KCs sprayed into the wounds remained within the FG and sometimes formed a
monolayer by day 14. AlloDerm, ADM, and
Integra became vascularized and infiltrated with fibroblasts within 7-14
days. AlloDerm and ADM became integrated
into the wound by 28 days (figure 4) whereas Integra underwent extensive
breakdown coincident with the appearance of numerous multinucleated giant
cells. Dermagraft formed a covering over
the wound that did not dissolve or remodel and did not promote or permit the
development of a neodermis. Dermalogen
was extensively resorbed post-operatively and remained highly disorganized such
that poor healing resulted. In wounds
lacking any dermal substitute, KCs tended to migrate toward the wound base but
did not form a monolayer. The lack of a
dermis resulted in extensive contraction of these wounds.
Laminin Immunostaining and
Vessel Counts
Vessel counts were
performed on immunostained sections from biopsies taken on days 14 and 28
(figures 5 and 6). Six different regions
were scored for each section: wound center superficial (WCS) and deep (WCD),
wound margin superficial (WMS) and deep (WMD), normal peripheral dermis
superficial (NPS) and deep (NPD) (Figure 7).
The primary control in this study was normal skin from unwounded animals
(CT) which was analyzed for vessel counts in the superficial and deep
regions. For each of the groups, the
number of vessels in the normal peripheral (NP) tissue flanking the wound was
an additional internal control. These
two controls (CT and NP) were compared (figure 8) for each of the dermal
substitute groups and were found to be not significantly different (ANOVA,
p=0.23). A group of animals treated with
allogenic or autogenic skin grafts was included. Gross wound healing was very good in this
group but immunostaining labeled pre-existing and newly formed vessels
indistinguishably.
On day 14, the Integra,
Dermagraft, and Dermalogen groups showed statistically significant differences
in the number of vessels in the WCS and WCD regions compared to CT skin (figure
9; ANOVA, p < 0.01). Wounds treated
with AlloDerm (for WCD) or ADM (for WCS) had significantly fewer vessels in the
WC than CT (Tukey test, p< 0.05). For
Dermalogen and Dermagraft, there were increased numbers of vessels in the wound
margin (p<0.01). By day 28 (figure
10), the vessel counts for each of the groups had normalized, with no
statistically significant differences between any of the groups and CT except
for AlloDerm, which was hypervascular in the WCS (p<0.01), and KC + FG and
Dermagraft which were hypervascular in the WMS (p<0.01).
In the superficial dermis, the number of vessels in
most groups approached the level of CT at day 28 (figure 11). However, the vascularity in the superficial
dermis with AlloDerm between days 21 and 28 underwent a striking increase
resulting in hypervascularity (p < 0.01).
Dermalogen, ADM, and Integra showed limited vascularity in the
superficial dermis on day 14 (p<0.01, p<0.05, and p<0.01,
respectively) until day 28, when the number of vessels was similar to normal
dermis. In contrast, hypervascularity
was seen in the superficial dermis for the Dermagraft group beginning at day 7
(data not shown) with a peak at day 14 (p < 0.05) and normalization by day
28. Vascularity in wounds treated with
FG only was similar to CT from day 7 to day 28.
In the deep dermis, the patterns of vessel ingrowth
were similar to those described above for the superficial dermis (figure 12). At
day 14, there was a statistically significant elevation in vessel number for
Dermagraft and Integra (p<0.01), with very little vascularity in the
AlloDerm (p<0.05) or Dermalogen (p<0.01) groups. All of the wounds approached normal levels of
vascularity by day 28, with no statistically significant differences from CT.
Automated Vessel Counts
Image analysis was used to evaluate
the impact of KCs on vascularization of wounds treated with dermal substitutes
and FG. At day 28 in the wound center,
significantly more blood vessels were present when KCs were included (p=0.027,
paired t-test) (figure 13). In addition,
the average area per blood vessel (µm2/ vessel) was calculated for
these groups. In CT skin, the average
vessel size was 300 µm2. In
the groups without KCs, the average blood vessel size was 360 µm2. In the groups sprayed with KCs + FG, the
average blood vessel size was equal to that in normal CT mouse skin at 300 µm2.
DISCUSSION
Angiogenesis is an integral part of normal wound
healing. Blood vessels deliver oxygen,
nutrients and inflammatory cells into the wound, and also remove necrotic
tissue from the area. Appropriate
angiogenesis is a complex process that includes endothelial cell division, selective
degradation of vascular basement membrane and of surrounding extracellular
matrix, and endothelial cell migration.31 Each step requires an
appropriate balance between activators or growth factors and inhibitors.
32 In addition, the level of
organization of the extracellular matrix plays a key role in the regulation of
neovascularization, in that it provides support for migrating endothelial cells
and acts as a reservoir for growth factors.33 Ideally, full-thickness wounds undergo an
initial phase of vigorous angiogenesis that is later followed by vessel
regression, such that the final pattern of vascularization is similar to that
of normal skin.32
However, no studies have been
published comparing wound healing and particularly angiogenesis using the
different commercially-available dermal substitutes. Most studies compare one substitute to
split-thickness skin grafting and most use subjective criteria for evaluating
the results.15 Thus, it is difficult to objectively determine which
dermal substitutes are most useful in the treatment of full-thickness
wounds. Within this context, there are
few studies which have examined the process of neovascularization in wounds
implanted with dermal substitutes. We
hypothesized that wounds implanted with dermal substitutes composed of
undenatured, allogenic extracellular matrix, such as ADM and AlloDerm, wound be
readily incorporated and have a final level of vascularization more similar to
normal skin than dermal substitutes composed of synthetic, xenogenic, or
denatured substances, such as Integra, Dermalogen, and Dermagraft. To a great extent, this hypothesis was
confirmed by the data presented here.
Grossly, wounds implanted with ADM, AlloDerm, or
Integra demonstrated less wound contraction (about 40% by day 28) and better
cosmetic results than did Dermagraft, Dermalogen, or KC + FG only. Our findings indicate that of the dermal
substitutes with native compositions, ADM implants achieved a normal level of
vascularization gradually over the 28 day period. On the other hand, AlloDerm which has a
composition similar to ADM, underwent a gradual increase in the number of
vessels but was hypervascular in the superficial dermis at day 28. For the synthetic dermal substitutes,
vascularization proceeded gradually and approached normal at day 28, with the
exception of Dermagraft which was highly vascularized at days 7 (data not
shown) and 14.
The results for Integra were noteworthy in that
despite its denatured, xenogenic composition, a controlled pattern of vessel
ingrowth conducive to improved wound healing was seen. However, the collagen-GAG matrix of Integra
has been specifically designed to have the necessary pore size essential to
ensure adequate microvascularization of the neodermis.34-37 Wounds in the Dermagraft group demonstrated
extensive granulation which may to some extent be attributable to the presence
of fibroblasts and therefore vascular endothelial growth factor (VEGF) in the
matrix. Similarly, when fibroblasts are
added to other dermal substitutes, such as de-epidermized dermis, enhanced
wound vascularization has been shown.38 However, in our study increased
vascularization during the healing process does not appear to improve wound healing. We also observed significantly increased
amounts of vascularization in wounds treated with KCs + FG in comparison to
wounds which received FG alone. KCs are
known to produce VEGF which is a potent inducer of angiogenesis.31 In wounds treated with KCs that overexpress
VEGF, decreased wound contraction, improved tissue development, and increased
vascular density are seen in the dermis.39 Nonetheless, in the present study the final
outcome in wounds treated with only KCs + FG or FG alone was clearly scarring
with extensive wound contraction. Thus,
the supranormal increase in vascular density present during the course of
healing correlated with increased
wound contraction.
Overall, there seems to
be an optimal level of vascularization in healing wounds, where either
increased or decreased levels lead to suboptimal wound healing. A certain level of vascularity of any dermal
substitute is required for the subsequent take of STSG, CEA, or other KC
preparations. The observed differences
between the healing seen with the dermal substitutes studied here will depend
on a number of factors including differences in levels of tissue oxygenation,
in the growth factor milieu, and in the structure and composition of the
extracellular matrices. Dermagraft
underwent extensive early granulation, whereas AlloDerm and Dermalogen
underwent delayed vascularization.
AlloDerm, Integra, and ADM underwent progressive vessel ingrowth that
seemed to be conducive to normal dermal regeneration, reepithelialization, and
limited wound contraction. These results
indicate that the rate and final extent of vascularization are important
determinants of the efficacy of dermal substitution for treating full-thickness
wounds. By evaluating wound contracture,
epithelialization, and angiogenesis in this nude mouse model, the value of novel
biomaterials as dermal substitutes may be predicted.
In the treatment of
chronic wounds, stimulation of angiogenesis effectively promotes wound closure in
patients with diabetes or peripheral vascular disease. However, in the treatment of acute
full-thickness wounds, hypervascularity or granulation must be limited to
reduce contraction and scarring. As seen
here, AlloDerm, ADM, and Integra demonstrated the desired pattern of
vascularization whereas other materials tested tended to become hypervascularized. Thus, we may expect that the use of AlloDerm,
ADM, or Integra should lead to improved healing of full-thickness wounds in
patients.
ACKNOWLEDGMENTS
The
authors would like to thank Paolo
Gattuso, MD for
giving them access to the ChromaVision system and Christopher Valadez and
ChromaVision technical support for their assistance in using the system.
REFERENCES
1.
Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung
WK. Successful use of a physiologically acceptable artificial skin in the
treatment of extensive burn injury. Annals
Surg. 1981; 194: 413-427.
2.
Hansbrough JF, Boyce ST, Cooper ML, Foreman T.
Burn wound closure with cultured autologous keratinocytes and fibroblasts
attached to a collagen-glycosaminoglycan substrate. JAMA. 1989; 262: 2125-2130.
3.
Boyce
ST, Greenhalgh DG, Kagan RJ, et al. Skin anatomy
and antigen expression after burn wound closure with composite grafts of
cultured skin cells and biopolymers. Plast
Reconstr Surg. 1993; 91: 632-64.
4.
Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T.
Living tissue formed in vitro and accepted as skin-equivalent tissue of
full-thickness. Science. 1981;
211:1052-1054.
5.
Heimbach D, Luterman A, Burke J, et al.
Artificial dermis for major burns. Annals
Surg. 1988; 208: 313-319.
6.
Matsuda K, Suzuki S, Isshiki K, et al. A bilayer
"artificial skin" capable of sustained release of an antibiotic. Brit J Plast Surg. 1991; 44: 142-146.
7.
Cooper ML, Hansbrough JF. Use of a composite skin
graft composed of cultured human keratinocytes and fibroblasts and a
collagen-GAG matrix to cover full-thickness wounds on athymic mice. Surgery. 1991; 109: 198-207.
8.
Hansbrough JF, Morgan J, Greenleaf G. Evaluation
of Graftskin composite grafts on full-thickness wounds on athymic mice. J Burn Care Rehabil. 1994; 15: 346-353.
9.
Hansbrough JF, Morgan J, Greenleaf G, Bartel R.
Composite grafts of human keratinocytes grown on a polyglactin mesh-cultured
fibroblast dermal substitute function as a bilayer skin replacement in
full-thickness wounds on athymic mice. J
Burn Care Rehabil. 1993; 14: 485-494.
10.
Matouskova E, Vogtova D, Konigova R. A recombined
skin composed of human keratinocytes cultured on cell-free pig dermis. Burns.
1993; 19: 118-123.
11.
Takami Y, Matsuda T, Yoshitake M, Hanumadass M,
Walter RJ. Dispase/detergent treated dermal matrix as a dermal substitute. Burns. 1996; 22: 182-190.
12.
Teepe RGC, Kreis RW,
Korbrugge EJ, et al. The use of cultured autologous epidermis in the
treatment of extensive burn wounds. J
Trauma. 1990; 30: 269-275.
13.
Nanchahal J, Ward CM. New grafts for old? A review of alternatives to autologous skin. Brit J Plast Surg. 1992; 45: 354-363.
14.
Shakespeare P. Burn wound healing and skin
substitutes. Burns. 2001; 27: 517-522.
15.
Kearney JN.
Clinical evaluation of skin substitutes. Burns.
2001; 27: 545-551.
16.
Balasubramani M, Kumar TR, Babu M. Skin
substitutes: a review. Burns. 2001;
27: 534-544.
17.
Boyce ST. Design principles for composition and
performance of cultured skin substitutes. Burns.
2001; 27: 523-533.
18.
Rue LW, Cioffi WG, McManus WF, Pruitt BA. Wound
closure and outcome in extensively burned patients treated with cultured
autologous keratinocytes. J Trauma. 1993; 34: 662-667.
19. Gallico GG, O’Connor NE, Compton CC, Kehinde O,
Green H. Permanent coverage of large
burn wounds with autologous cultured human epithelium. NEJM.
1984; 311: 448-451.
20. Cohen
M, Bahoric A, Clarke HM. Aerosolization of epidermal cells with fibrin glue for
the epithelialization of porcine wounds with unfavorable topography. Plast Reconstr Surg. 2001; 107:
1208-1215.
21. Currie LJ, Martin R, Sharpe
JR, James SE. A comparison of
keratinocyte cell sprays with and without fibrin glue. Burns.
2003; 29: 677-685.
22. Horch RE, Bannasch H, Kopp J, Andree C, Stark GB. Single-cell suspensions of cultured human
keratinocytes in fibrin glue reconstitute the epidermis. Cell
Transplant. 1998; 7: 309-317.
23. Hunyadi J, Farkas B, Bertenyi
C, Olah J, Dobozy A. Keratinocyte
grafting: a new means of transplantation for full-thickness wounds. J
Dermatol Surg Onc. 1988; 14: 75-78.
24. Ronfard V, Rives J-M, Neveux
Y, Carsin H, Barrandon Y. Long-term
regeneration of human epidermis on third degree burns transplanted with
autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000; 70: 1588-1598.
25.
Fraulin FOG, Bahoric DVM, Harrop AR, Hiruki T, Clarke HM. Autotransplantation of epithelial cells in
the pig via an aerosol vehicle. J Burn Care Rehabil 1998; 19: 337-345.
26.
Navarro FA, Stoner ML, Lee HB, Park CS, Wood FM,
Orgill DP. Melanocyte repopulation in full-thickness wounds using a cell spray
apparatus. J Burn Care Rehabil. 2001;
22: 41-46.
27. Navarro
FA, Stoner ML, Park CS, Huertas JC, Lee HB, Wood FM, Orgill DP. Sprayed
keratinocyte suspensions accelerate epidermal coverage in a porcine microwound
model. J Burn Care Rehabil. 2000;. 21: 513-518.
28.
Jiao XY, Kopp J, Tanczos E, Voigt M, Stark
GB. Cultured keratinocytes suspended in fibrin glue to
cover full-thickness wounds on athymic nude mice: comparison of two brands of
fibrin glue. Eur J Plast Surg. 1988; 21: 72-76.
29.
Chester DL, Balderson DS,
Papini RPG. A
review of keratinocyte delivery to the wound bed. J Burn Care
Rehabil. 2004; 25: 266-275.
30.
Currie LJ, Sharpe JR, Martin R.
The use of fibrin glue in skin grafts and tissue-engineered skin
replacements: a review. Plast Reconstr Surg. 2001; 108:
1713-1726.
31.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular
endothelial growth factor, microvascular hyperpermeability, and
angiogenesis. Am J Pathol. 1995; 146: 1029-1039.
32.
Lingen MW.
Role of leukocytes and endothelial cells in the development of
angiogenesis in inflammation and wound healing.
Arch Pathol Lab Med. 2001;
125: 67-71.
33.
Li J, Zhang Y-P, Kirsner RS.
Angiogenesis in wound repair: angiogenic growth factors and the
extracellular matrix. Microsc Res Tech. 2003; 60: 107-114.
34.
Burke JF. Observations on the
development of an artificial skin: presidential address, 1982 American Burn
Association Meeting. J Trauma. 1983;
23: 543-551.
35. Moiemen NS, Staiano JJ, Ojeh, NO, Thway
Y, Frame JD. Reconstructive surgery with a dermal regeneration template:
clinical and histologic study. Plast Reconstr Surg. 2001; 108: 93-103.
36. Stern R, McPherson M,
Longaker MT. Histologic study of
artificial skin used in the treatment of full-thickness thermal injury. J Burn Care Rehabil. 1990; 11: 7-13.
37. Sheridan RL, Hegarty M, Tompkins RG, Burke JF. Artificial skin in massive burns-results to
ten years. Eur J Plast Surg. 1994; 17:
91-93.
38. Erdag G, Sheridan RL.
Fibroblasts improve performance of cultured composite skin substitutes on
athymic mice. Burns. 2004; 30: 322-328.
39. Supp DM, Boyce ST. Overexpression of vascular endothelial growth
factor accelerates early vascularization and improves healing of genetically
modified cultured skin substitutes. J Burn Care Rehabil. 2002; 23: 10-20.
FIGURES
Figure
1
Wound
area over the 28 day study period for each of the dermal substitutes. Values are normalized against the size of
each wound on the day of surgery. Dashed
horizontal line represents starting wound area in normal unwounded mouse skin.
Figure
2
Gross
appearance of wounds on the day of surgery shows the structural differences
between the dermal substitutes.
Full-thickness wounds (2 x 2 cm) were created, followed by implantation
of the dermal substitute and sprayed FG and KCs. Nylon sutures were placed at all four corners
of the wound to mark the original wound size.
Figure
3
Gross
appearance of wounds on post-operative day 28 shows the healed wounds and the
degree of contraction. Sutures mark the
corners of the original wound. Note the
extensive contraction of wounds treated with KC + FG only and Dermalogen in
comparison to the minimal contraction observed with use of Integra, AlloDerm,
and ADM. Dermagraft-implanted wounds showed poor epithelialization and
incorporation into the wound with contraction limited only as long as the
implant was retained.
Figure
4
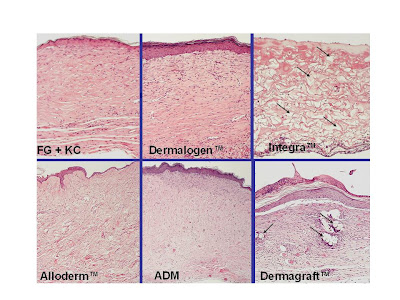
H&E
stained paraffin cross-sections of day 28 biopsies for each of the dermal
substitute groups. The Integra specimen
shows scattered KCs on the surface of the FG which is overlying the Integra and
numerous small islands of residual gel within the developing neodermis
(arrows). The Dermagraft specimen shows
synthetic fibers that have been incorporated into the wound (arrows). The AlloDerm and ADM specimens are
epithelialized and show a structured dermal matrix populated with fibroblasts,
blood vessels, and other connective tissue components. Magnification bar = 200 μm
Figure
5
Cross-sections
of wound biopsies immunostained for laminin showing the wound center for
Integra and AlloDerm on day 14. Vessels
can be clearly identified (arrows) within the deep dermis for Integra. The superficial region of the Integra implant
and the entire AlloDerm implant are evident and show few vessels. Magnification bars = 100 μm
Figure
6
Cross-sections
of wound biopsies immunostained for laminin showing the wound center for
Integra and AlloDerm on day 28. AlloDerm
has numerous vessels scattered throughout the dermis as does Integra. Note the numerous large and small cavities in
the Integra implant. In life, these
cavities held the collagen-chondroitin sulfate colloid that comprises
Integra. Magnification bars = 100 μm
Laminin-stained
paraffin cross-section of Integra on day 28 illustrating the different regions
in which vessel counts were performed.
Three different zones were identified: Normal
peripheral (NP) tissue, wound margin (WM), and wound center (WC). Within each zone, counts were performed in
the superficial regions (S), the area directly below the epidermal basement
membrane and in the deep regions (D).
Note that the normal peripheral zones that were actually counted were
further away from the wound margin than shown (>300 μm). In total, six different regions (NPS, NPD,
WMS, WMD, WCS, and WCD) were analyzed for each section. Magnification bar = 150 μm
Stacked
bar graph showing blood vessel counts in superficial (sup) and deep regions of
normal peripheral tissue for each dermal substitute on days 14 and 28
post-surgery. The dotted line represents
the total number of blood vessels (i.e., superficial + deep) seen in control
normal mouse skin (CT). Error bars
represent standard error of the mean.
One-way ANOVA did not show any significant differences between groups
and CT, p = 0.238
Stacked
bar graph shows blood vessel counts (mean ± SEM) in the superficial (sup) and
deep regions of the wound center and wound margin on day 14 post-surgery for
each dermal substitute. The dotted line
represents the total number of blood vessels (i.e., superficial + deep) in
control normal mouse skin (CT). Data
were analyzed using one-way ANOVA with Tukey post-tests.
Figure
10
Stacked
bar graph shows blood vessel counts (mean ± SEM) in the superficial (sup) and
deep regions of the wound center and wound margin on day 28 post-surgery for
each dermal substitute. The dotted line
represents the total number of blood vessels (i.e., superficial + deep) in
control normal mouse skin (CT). Data
were analyzed using one-way ANOVA with Tukey post-tests.
Figure
11
Graph
depicting changes in vessel numbers (mean ± SEM) in the superficial dermis at the center of the wound during the 28-day
study period. The dashed horizontal line
represents the vascular counts in normal mouse skin (CT). Note the great elevation in vessel numbers
for Dermagraft implants especially at day 14.
By day 28, vascular counts were similar to CT for most of the
groups. Data were analyzed using one-way
ANOVA with Tukey post-tests.
**p
< 0.05 for Dermalogen, Integra, or ADM vs CT
*p < 0.01 for AlloDerm on day 28 and for
Dermagraft on day 14 vs CT
Figure
12
Graph
depicting changes in vessel numbers (mean ± SEM) in the deep dermis at the center of the wound during the 28-day study
period. The horizontal dashed line
represents the vascular counts in normal mouse skin (CT). Note the great elevation in counts for
Dermagraft implants especially at day 14.
By day 28, vascular counts were similar to CT for all groups. Data were analyzed using one-way ANOVA with
Tukey post-tests.
**p
< 0.05 for AlloDerm vs CT
*p < 0.01 for Dermagraft, Integra, or
Dermalogen vs CT
Figure
13
Vessel
counts from ChromaVision ACIS system at the wound center for wounds treated
with dermal substitute + FG + KC versus wounds treated with dermal substitute +
FG alone on day 28. The horizontal
dashed line represents the vascular counts in normal mouse skin. Overall, there are significantly (p=0.027,
paired t-test) more blood vessels seen when KCs are present.
FYI. The content on this blog is copyright protected by the author. Feel free to read, copy, and disseminate the studies described here, but please indicate the origin of the work by citing this blog URL as an electronic web citation when it is appropriate to recognize and attribute the work of others.
FYI. The content on this blog is copyright protected by the author. Feel free to read, copy, and disseminate the studies described here, but please indicate the origin of the work by citing this blog URL as an electronic web citation when it is appropriate to recognize and attribute the work of others.











Hi, I was simply checking out this blog and I really admire the premise of the article sigma antibody
ReplyDeletegreat blog..
ReplyDeleteundenatured type ii collagen peptide