This is the second of 2 articles detailing wound healing studies done in nude mice implanted with different biological materials. Full thickness skin wounds were created on the dorsum and then different dermal replacements were implanted. Healing was followed for 28 days and very detailed histological and immunostaining evaluations of the wounds were performed to quantify the healing process. Most studies of wound healing use simple or highly subjective measures of healing, but one of the main goals of these studies was to use objective criteria to quantify healing and to do so in fine detail.
Below is the full text of this article and also a PDF link of higher quality.
Wound Neovascularization and Dermal Substitutes in Nude Mice
Neovascularization of Wounds Treated
with Dermal Substitutes in Nude Mice
by
Michele M. Loor, MD, Anh-Tuan Truong, MD, Barbara A.
Latenser, MD 1,2,3 ,
Dorion E. Wiley, MD 1,2, and Robert J.
Walter, PhD 1,2
1 Sumner L. Koch Burn Center, Department of Trauma, John
Stroger Jr. Hospital of Cook
County &
2 Department of General Surgery, Rush
University Medical
Center, Chicago, Illinois
3 Current address: Director, Burn
Unit, University of Iowa, Iowa City, IA
Address Correspondence to:
Robert J. Walter, PhD
Department of Trauma, Suite 1300
John Stroger Jr. Hospital of Cook County
1900 West Polk Street
Chicago, IL 60612
Phone: 312.864.0578
Email: rwalter@rush.edu
ABSTRACT
Background: Dermal substitutes implanted into
full-thickness skin wounds reduce wound contraction, improve cosmesis, and
improve function. These effects depend
upon the development of optimal vascularization of the dermal substitute.
Study Design: Full-thickness skin wounds were created on
the dorsum of nude mice. A dermal matrix
(Integra®, AlloDerm®, acellular dermal matrix, Dermalogen®, or Dermagraft®) was
implanted and covered by a mix of fibrin glue (FG) and human keratinocytes. Wound healing was observed for 4 weeks. Biopsies were immunostained for laminin
followed by blood vessel quantitation using image processing. Vessels were quantified in the superficial
and deep dermis from the wound center, wound margin, and from peripheral unwounded
dermis.
Results: Extensive vascularity was seen at day 28 in implanted
Dermagraft® and Dermalogen®. AlloDerm®,
ADM, and Integra® showed slower vessel ingrowth from the wound base and margins
and, by day 28, showed diminished wound contraction. The average vessel size in
wounds treated with Integra was greater than normal at both days 14 and 28.
Conclusions: Dermagraft® and Dermalogen® underwent
extensive granulation whereas AlloDerm®, Integra®, and ADM showed a more
controlled, progressive vessel ingrowth.
For AlloDerm® and ADM, this pattern was associated with reduced wound
contraction and increased epithelialization.
Keywords:
neovascularization, dermal substitutes, wound healing, nude mice, fibrin glue
Abbreviations: ADM:
acellular dermal matrix, FG: fibrin glue, KC: keratinocytes, ACIS: automated
cellular imaging system, WC: wound center, WM: wound margin, NP: normal
peripheral tissue
INTRODUCTION
The
treatment of full-thickness skin wounds poses a significant clinical
challenge. An open wound not only
provides a portal of entry for microorganisms, but it also allows vital fluid
and electrolytes to escape. The primary objective in treating an open wound is,
therefore, early coverage. Secondarily,
healed wounds must be optimized for
function and appearance.
Full-thickness wounds lack a dermis and dermis regenerates poorly or not
at all. As a result, these wounds heal
slowly with significant scarring and contracture. Various synthetic collagen-based dermal
matrices are now available for use in wounds.1-11 Dermal replacements have space-filling
properties and are intended to provide rapid wound coverage. They must also
permit host cell infiltration and controlled neovascularization so that the
dermal substitute may be quickly incorporated into the wound and subsuquently
remodelled to form dermis that is as similar to unwounded dermis as
possible. However, the efficacy of the
available dermal substitutes materials in the treatment of full-thickness
wounds has, with the exception of our recent studies12,13, seldom
been carefully compared.
In
most cases, placement of a dermal matrix is later followed by split-thickness
skin grafting to provide definitive wound closure. The use of cultured autologous keratinocytes (KCs) has been
studied extensively as an alternative to autografting, particularly in patients
with greater than 40% total body surface area wounds who have limited donor
site availability.14-21 Several methods of KC delivery to wounds are
available including combination with a fibrin sealant 22-26 and
application via a spray apparatus.27-32
We have developed a spray method for applying KCs suspended in
fibrin sealant to the wound surface. In vitro and in vivo tests show that the viability and proliferative potential
of the sprayed KCs remains very high.
Neovascularization
is a key step in wound healing. It is a
complex process that is dependent upon appropriate interactions between the
extracellular matrix, the migrating endothelial cells, and a number of growth
factors. Dermal substitutes composed of
native (undenatured), allogenic extracellular matrix such as acellular dermal
matrix (ADM) and AlloDerm® become readily vascularized, complement healing, and
reduce contractive scarring in a variety of wound types. We hypothesized that the mode and rate of
neovascularization during the process of wound healing in the presence of ADM
or AlloDerm® may be important determinants in final wound resolution. The process of wound vascularization may be
significantly altered with materials such as Integra®, Dermalogen®, and Dermagraft®
which contain synthetic, highly modified, or denatured substances that may
negatively affect wound resolution.
To
evaluate this, dermal substitutes in conjunction with FG and KCs were
introduced into full-thickness wounds on the dorsum of nude mice. Paraffin-embedded sections from weekly
biopsies were immunostained for laminin, an antigen found in the endothelial
basement membrane, to assess and compare vascularization in these healing
wounds. The progress of wound healing
and particularly dermal neovascularization was evaluated quantitatively over a
period of four weeks based on digital imaging and analysis software.
METHODS AND MATERIALS
The
following materials were used:
§
Tisseel® (Baxter
Health, Deerfield, IL) is a two component system in which
fibrinogen, calcium, thrombin, and a protease inhibitor are combined and
dispensed onto a wound or other surface to form a fibrin clot.
§
Integra®
(Ethicon, Somerville, NJ) is a bilayer artificial skin composed of a “dermal”
layer of bovine collagen gel cross-linked with shark chondroitin-6-sulfate and
an “epidermal” layer of polysiloxane polymer (which was removed for this
study). Integra® is indicated for
partial-thickness wounds, but is being used increasingly for full-thickness
wound treatment.
§
AlloDerm®
(Lifecell Corp., Branchburg,
NJ) is an undenatured collagen
matrix derived from human skin that is treated to remove most of the cellular
components.
§
Acellular Dermal Matrix (ADM) is a native
dermal collagen matrix derived from human skin that is treated to remove all
cellular components. The preparation and
characterization of this matrix material has been described previously 11,33.
Briefly, human cadaver skin was treated with Dispase to remove epithelial cells
and then Triton-X detergent to remove all residual cells and cellular
debris.
§
Dermalogen®
(Collagenesis Corp, Beverly,
MA) is a powdered human dermal
collagen matrix that has been treated to remove some cellular components and is
used primarily for aesthetic surgery.
§
Dermagraft® (Smith
& Nephew, Largo,
FL) is comprised of a woven bioabsorbable polymer on and in which human dermal
fibroblasts are grown and then devitalized.
This material is indicated for treating full-thickness wounds.
Animals and Surgery
NIH
homozygous male nude mice, 4 weeks of age (Taconic, Germantown, NY)
were used. All surgical interventions were performed in the John H. Stroger,
Jr. Hospital of Cook County Animal Facility using protocols approved by the
IACUC. Preoperative Kanamycin (25U/kg
IM) was administered to the animals and ketamine/ xylazine was used for
anesthesia. Under aseptic conditions, 2
cm x 2 cm full-thickness wounds were excised down to the muscle fascia and then
implanted with: 1) Integra®, 2) AlloDerm®, 3) ADM, 4) Dermalogen®, 5) Dermagraft®,
6) KCs + FG only, or 7) FG only. Each
group was comprised of at least 6 mice, three of which underwent weekly
biopsies. In the groups of mice that
received dermal substitutes, the substitute was cut to size and sutured into
the wound using 4-0 nylon sutures. In
the Dermalogen® group, 0.75 cc of the viscous suspension was placed into the
wound. Animals in groups 1-6 received
human KCs sprayed onto the dermal substitutes or wound surface in combination
with FG. KCs (4 X 105/ cc)
were suspended in the thrombin component of Tisseel® and were sprayed such that
the final number of KCs applied to each wound was 2 X 105 in 1.0 cc
of FG. All defects were covered with a
semi-permeable adhesive film (Op-Site, Smith & Nephew, Largo, FL), Xeroform
(Sherwood Medical, St. Louis, MO), dry cotton gauze (Adaptic, Johnson &
Johnson, New Brunswick, NJ), and finally with a fine stainless steel mesh fixed
to the animals’ back with skin sutures.
This last was used to prevent the wounds from being disturbed by chewing
or scratching. The dressings were
inspected daily. Biopsies were performed
on designated animals at 7, 14, 21, and 28 days post-surgery. The harvested
biopsies were fixed in 10% buffered formalin, paraffin embedded, and sections
stained with H&E or immunostained.34
Human KC Culture and Preparation
for Spraying
Human
KCs, Epilife culture medium, supplements, and transfer solutions were obtained
from Cascade Biologics (Portland,
OR). Cells were grown in 75 cm2 flasks
from expanded frozen stocks stored after the second passage. After 7-10 days of proliferation and growth,
flasks containing 50-80% confluent KCs were washed, trypsinized briefly to
release cells from the substrate, trypsin was neutralized, and the suspension
centrifuged at 20xg for 5 min and 4ºC.
The supernatant was discarded and the cells resuspended in fresh Epilife
medium. Cells were mixed with the reconstituted thrombin/calcium component of
the fibrin sealant kit at a 1:1 dilution.
The fibrinogen component was also reconstituted and diluted 1:1 with
Epilife. This components was stored at
4ºC until being sprayed onto wounds in conjunction with the fibrinogen
component using a tuberculin syringe fitted with a spray head as indicated
above.34
Immunostaining
Tissue
sections were deparaffinized and antigen retrieval was performed by incubating
specimens in pepsin (1mg/ ml 0.01N HCl) at 37ºC for 2 hours. Following this, nonspecific binding was
blocked using 1% bovine serum albumin and sections were then incubated in
rabbit anti-mouse laminin IgG (Sigma, St. Louis, MO) at a 1:10 dilution
followed by HRP-conjugated goat anti-rabbit IgG secondary antibody (Cappel,
Irvine, CA) at a dilution of 1:100.
Reaction product was generated using a Vector DAB/peroxide Developing
Kit (Vector, Burlingame, VT) according to the manufacturer’s specifications
with a 10 min developing time and specimens were counterstained with
hematoxylin QS (Vector, Burlingame, VT).
Normal skin from previously unwounded mice was also immunostained for
laminin to serve as a control. Laminin
staining marked the basement membrane of epidermis, nerve, muscle, and
vessels. Within the dermis,
immunostained structures showing an open lumen were counted as blood
vessels. These were readily
distinguished from the other positively stained structures by their location
and morphology.
Data Analysis
Wound
characteristics were measured grossly and histologically. Gross observations were made at days 7, 14,
21, and 28 post-surgery. Wound
contraction and degree of epithelialization were measured using UTHSCSA
ImageTool software in conjunction with digital photographs of wounds Vessel quantification was performed using the
ChromaVision (San Juan Capistrano,
CA) Automated Cellular Imaging
System (ACIS) in conjunction with microvascular density (MVD) software. Vessels were counted in the superficial
(papillary) dermis, i.e., the region of dermis directly beneath the epidermis,
and in the deep (reticular) dermis, i.e., immediately above the hypodermis. Three different zones of each wound biopsy
were evaluated in this way: the wound center (WC), wound margin (WM) and
unwounded normal dermis (NP) peripheral to the wound margin. Zone selection was standardized by defining:
WC as the region equidistant from each wound margin; WM as the area directly
adjacent to unwounded tissue; and NP as the area with normal skin structures
and appendages at least 500 um away from the wound margin. Data obtained by
image analysis included the microvascular density (number of vessels/ mm2)
and the vessel area (µm2/ vessel) based on laminin
immunostaining. Results were analyzed
by one-way ANOVA with Tukey’s post tests.
RESULTS
Gross wound observations
Each of the dermal substitutes except Dermalogen® reduced the amount of
wound contraction as compared to wounds that received only sprayed KCs + FG
(figure 1). Thirty-five to 45% wound
contraction was observed in wounds implanted with AlloDerm®, Dermagraft®,
Integra®, or ADM at 28 days post-operatively.
Greater contraction (60%) was observed in the Dermalogen® and KCs + FG
groups (figure 2). Dermagraft®-implanted
wounds showed poor epithelialization and poor incorporation into the wound with
contraction limited only as long as the implant was retained. Often Dermagraft® implants underwent partial
or total spontaneous dehiscence and were rejected from the wound.
Laminin Immunostaining and Vessel Counts
Vessel counts were performed on immunostained sections from biopsies
taken on days 14 and 28 (figures 2 and 3).
Six different regions were scored for each section: wound center,
superficial and deep; wound margin, superficial and deep; normal peripheral
dermis, superficial and deep. For some
parts of the analysis superficial and deep counts were combined to yield total
counts representative of each region.
The primary control in this study was skin from previously unwounded
animals (CTL) for which vessel counts in the superficial and deep regions were
performed. For each of the groups,
additional internal controls were included by analyzing the number of vessels
in the normal peripheral tissue flanking the wound. For most of the experimental groups, this
tissue exhibited vascularity similar to that of CTL skin.
During
the 28 day study period, the number of vessels seen in the superficial dermis
rose from low levels at day 14 to supernormal levels at day 28 for Dermagraft®
(p<0.05 vs CTL, one-way ANOVA,Tukey’s post tests) and Dermalogen® (figure
4). Hypervascularity was seen in the
superficial dermis for the KC + FG group beginning at day 14 with little change
in vascularity between days 14 and 28.
AlloDerm®, ADM, and Integra® all showed minimal vascularity in the
superficial dermis at day 14 (p<0.01 for ADM and AlloDerm vs CTL, p<0.05
for Integra vs CTL). By day 28, the
number of vessels in each of these groups approached the normal level but still
remained somewhat hypovascular.
Similar
patterns were seen for the number of vessels in the deep dermis over time
(figure 5). For AlloDerm®, there was
limited vessel ingrowth in the deep dermis at day 14 (p<0.05 vs CTL) and low levels at day 28. The Integra® and ADM groups demonstrated an
initial rise in vascularity at day 14 followed by a decrease in vessel number
on day 28, such that both groups were ultimately hypovascular. In contrast, the Dermagraft®, Dermalogen®,
and KC + FG groupswere hypervascular at
day 28. With regard to total vascularity
(superficial + deep) at day 28, some of the dermal substitute groups were clearly
hypervascular (Dermagraft®, p<0.001, and Dermalogen®, p<0.05) while others
were hypovascular (Integra®, AlloDerm®, and ADM) compared to normal skin (CTL)
(table 1).
Vessel Area
The
average area per blood vessel was also determined using the ACIS system for
vessels present in the superficial and deep regions of the wound center. The average vessel area in control normal
mouse dermis (CTL) was used as the standard to which the values for implanted
skin substitutes were compared. In the superficial dermis, larger than normal
vessels were seen at day 14 for Integra (p<0.001 vs CTL), KC + FG, and
AlloDerm. In contrast, Dermagraft,
Dermalogen, and ADM had smaller than normal vessels at day 14 (p>
0.05). By day 28, vessel caliber for
each of the groups approached normal with no significant differences from CTL,
with the exception of Integra, which had persistently large vessels in the
superficial region (p<0.01) (figure 6).
This same feature of large caliber vessels was also seen in the deep
dermis of wounds treated with Integra at days 14 (p<0.01) and 28 (p<0.05)
(figure 7). In all other groups, vessel
size was similar to CTL at both time points.
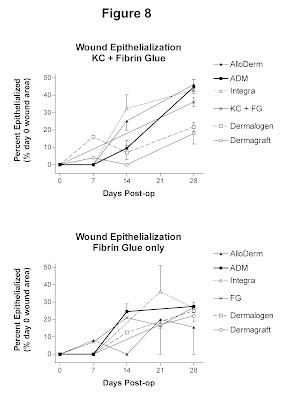
Degree of Epithelialization
Digital
imaging and analysis were used to determine the percent of the original wound
area which was reepithelialized at days 7, 14, 21, and 28 (figure 8). Wounds treated with AlloDerm, ADM, and Integra underwent approximately 40%
epithelialization by day 28. In
contrast, wounds treated with Dermalogen
or Dermagraft were less epithelialized (20% of original wound) at the
conclusion of the study. Wounds treated with KC + FG alone were 35%
epithelialized at day 28.
DISCUSSION
Angiogenesis
is integral to effective wound healing.
Blood vessels deliver oxygen, nutrients and inflammatory cells into the
wound and provide conduits for the removal of metabolic by-products and debris
from damaged tissue. Appropriate
angiogenesis is a complex process that involves endothelial cell division,
selective degradation of vascular basement membrane and of surrounding extracellular
matrix, and endothelial cell migration.35 The level of organization of the
extracellular matrix plays a key role in the regulation of neovascularization
in that it provides support for migrating endothelial cells and acts as a
reservoir for endothelial cell growth factors derived from the plasma or serum
and from infiltrating fibroblasts, leukocytes, and migrating KCs.36 It is thought that full-thickness wounds
should undergo an initial phase of vigorous angiogenesis that is later followed
by vessel regression, such that the final pattern of vascularization is similar
to that of normal skin.37
However, no studies have been published directly
comparing wound healing seen with different commercially-available dermal
substitutes. Thus, it is difficult to
objectively determine which dermal substitutes are most useful in the treatment
of full-thickness wounds. Within this
context, there are few studies which have examined the process of
neovascularization in wounds implanted with dermal substitutes. We hypothesized that wounds implanted with
dermal substitutes composed of undenatured, allogenic extracellular matrix such
as ADM and AlloDerm®, wound be readily incorporated and develop vascularization
more similar to normal skin than dermal substitutes composed of synthetic,
non-native, or denatured substances such as Integra®, Dermalogen®, and
Dermagraft®. To a large extent, this
hypothesis was confirmed by the data presented here.
Grossly,
wounds implanted with ADM, AlloDerm®, or Integra® demonstrated less wound
contraction losing about 40% of their original size by day 28 and better
cosmetic results than did Dermagraft®, Dermalogen®, or KC + FG only. Our findings indicate that, of the dermal
substitutes with native compositions, ADM and AlloDerm® underwent a gradual and
limited pattern of neovascularization and were ultimately somewhat hypovascular
at day 28. In terms of vessel size,
wounds treated with ADM and AlloDerm contained vessels of a caliber similar to
CTL at both day 14 and 28. For the
highly modified or synthetic dermal substitutes, Dermagraft® and Dermalogen®
(and KCs + FG alone), vascularization proceeded rapidly resulting in
hypervascularity by day 14 or day 28. The size of the vessels in these wounds
was also similar to CTL on days 14 and 28.
The
results for Integra® were noteworthy in that despite its denatured, highly
modified composition relative to normal dermis, it underwent a controlled
pattern of vessel ingrowth that appeared to be conducive to improved wound
healing. This seems to confirm the
previous claims that the collagen-GAG matrix of Integra® has been specifically
designed to have pore sizes that ensure adequate microvascularization of the
forming neodermis.38-40 Notably, wounds in the Integra group
contained vessels of significantly
larger areas than CTL at both days 14 and 28.
Upon further review of these specimens, it appears that they contain an
unusually large number of tortuous vessels that are oriented perpendicularly to
the wound surface. These features may
cause the vessel area calculations performed here to be skewed toward large
vessel areas since more vessels were cut in longitudinal or tangential section
due to their orientation in the tissue. This feature of Integra is likely
related to the design of the Integra matrix, with the orientation of the pores
allowing vessel growth in this configuration.
This result may be related to the improved wound healing observed with
the use of Integra and clinical observations showing its ability to support
overlying skin grafts.
Wounds in the Dermagraft® group demonstrated
extensive granulation which may, to some extent, be attributable to its content
of non-viable fibroblasts that may act as a source of vascular endothelial
growth factor (VEGF). Similarly, when viable
fibroblasts are added to de-epidermized dermis, enhanced wound vascularization
has been shown.42 However,
the present study illustrates that too much neovascularization can also result
in impaired wound healing. In the Dermalogen® and Dermagraft® groups
hypervascularity at day 28 correlated with increased
wound contraction and poor wound cosmesis.
Overall, there seems to be an optimal rate and final level of
vascularization in healing wounds, where either increased or decreased rates or
levels are associated with suboptimal healing.
These
results with Integra® and Dermagraft® also point up one important aspect of
wound healing could not be tested in this model. The model is insensitive to the xenogenic (in
this case, non-mouse) nature of some of the components of these dermal
substitutes because of the T cell immunodeficiency that characterizes nude
mice. Of course, it is this
immunodeficiency that makes a study such as this possible. Nonetheless, aspects of inflammation that
might be triggered in humans by the presence of allogenic or xenogenic
materials such as shark chondroitin sulfate, bovine collagen, or human
fibroblasts are not seen in this model.
Interestingly, the dermal substitutes that contain such materials do not
seem to evoke a strong immune response in humans.
Clinical
experience tells us that certain minimum amounts of wound vascularization must
be present and certain maximum amounts of granulation tissue may be tolerated
for implanted skin grafts to survive.
The present study shows that the composition of implanted dermal
substitutes can affect angiogenesis.
This will undoubtedly determine the level of oxygenation and the growth
factor milieu (EGF, FGF, PDGF, VEGF, etc.) in the dermis. Dermagraft® and Dermalogen® underwent
extensive granulation, whereas AlloDerm®, Integra®, and ADM underwent limited
vessel ingrowth that seemed to be conducive to the development of normal dermal
structure, reepithelialization, and minimal wound contraction. These data indicate that the rate and final
extent of vascularization are important determinants in the efficacy of dermal
substitution for the treatment of full-thickness wounds. Further efforts to achieve one-step
full-thickness wound closure will depend upon the use of dermal substitutes
that can vascularize rapidly enough to support overlying KCs or an ultra-thin
split-thickness graft, but will not induce overly abundant granulation tissue
formation. This is a realistic goal
using currently available biomaterials but further refinements are needed to
achieve optimal healing.
REFERENCES
1.
Burke JF, Yannas
IV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically
acceptable artificial skin in the treatment of extensive burn injury. Annals
Surg. 1981; 194: 413-427.
2.
Hansbrough JF,
Boyce ST, Cooper ML, Foreman T. Burn wound closure with cultured autologous
keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan
substrate. JAMA. 1989; 262: 2125-2130.
3.
Boyce ST, Greenhalgh DG, Kagan RJ, et al. Skin anatomy and
antigen expression after burn wound closure with composite grafts of cultured
skin cells and biopolymers. Plast Reconstr Surg. 1993; 91: 632-64.
4.
Bell E, Ehrlich
HP, Buttle DJ, Nakatsuji T. Living tissue formed in vitro and accepted as
skin-equivalent tissue of full-thickness. Science. 1981; 211:1052-1054.
5.
Heimbach D,
Luterman A, Burke J, et al. Artificial dermis for major burns. Annals Surg.
1988; 208: 313-319.
6.
Matsuda K, Suzuki
S, Isshiki K, et al. A bilayer "artificial skin" capable of sustained
release of an antibiotic. Brit J Plast Surg. 1991; 44: 142-146.
7.
Cooper ML,
Hansbrough JF. Use of a composite skin graft composed of cultured human
keratinocytes and fibroblasts and a collagen-GAG matrix to cover full-thickness
wounds on athymic mice. Surgery. 1991; 109: 198-207.
8.
Hansbrough JF,
Morgan J, Greenleaf G. Evaluation of Graftskin composite grafts on
full-thickness wounds on athymic mice. J Burn Care Rehabil. 1994; 15: 346-353.
9.
Hansbrough JF,
Morgan J, Greenleaf G, Bartel R. Composite grafts of human keratinocytes grown
on a polyglactin mesh-cultured fibroblast dermal substitute function as a
bilayer skin replacement in full-thickness wounds on athymic mice. J Burn Care
Rehabil. 1993; 14: 485-494.
10. Matouskova E, Vogtova D, Konigova R. A recombined skin
composed of human keratinocytes cultured on cell-free pig dermis. Burns. 1993; 19: 118-123.
11. Takami Y, Matsuda T, Yoshitake M, Hanumadass M, Walter
RJ. Dispase/detergent treated dermal matrix as a dermal substitute. Burns 1996;
22: 182-190.
12. Loor MM, Truong A-T, Kowal-Vern, A, et al.
Neovascularization during healing of wounds treated with dermal substitutes and
fibrin glue in nude mice. JACS. 2004;
199: S63.
13. Loor MM, Truong A-T, Latenser BA, Wiley DE, Watler RJ.
Healing and neovascularization of wounds implanted with dermal substitutes and
fibrin glue in nude mice. J Trauma. 2004; 57: 455.
14. Teepe RGC, Kreis RW, Korbrugge EJ, et al. The use of
cultured autologous epidermis in the treatment of extensive burn wounds. J
Trauma. 1990; 30: 269-275.
15. Nanchahal J, Ward CM. New grafts for old? A review of alternatives to autologous skin.
Brit J Plast Surg. 1992; 45: 354-363.
16. Shakespeare P. Burn wound healing and skin
substitutes. Burns. 2001; 27: 517-522.
17. Kearney JN. Clinical evaluation of skin substitutes. Burns.
2001; 27: 545-551.
18. Balasubramani M, Kumar TR, Babu M. Skin substitutes: a
review. Burns. 2001; 27: 534-544.
19. Boyce ST. Design principles for composition and
performance of cultured skin substitutes. Burns. 2001; 27: 523-533.
20. Rue LW, Cioffi WG, McManus WF, Pruitt BA. Wound
closure and outcome in extensively burned patients treated with cultured
autologous keratinocytes. J Trauma. 1993; 34: 662-66.
21. Gallico GG, O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with
autologous cultured human epithelium.
NEJM. 1984; 311: 448-451.
22. Cohen M, Bahoric A, Clarke HM. Aerosolization of
epidermal cells with fibrin glue for the epithelialization of porcine wounds
with unfavorable topography. Plast Reconstr Surg. 2001; 107: 1208-1215.
23. Currie LJ, Martin R, Sharpe JR, James SE. A comparison of keratinocyte cell sprays with
and without fibrin glue. Burns. 2003;
29: 677-685.
24. Horch RE, Bannasch H, Kopp J, Andree C, Stark GB. Single-cell suspensions of cultured human
keratinocytes in fibrin glue reconstitute the epidermis. Cell Transplant. 1998; 7: 309-317.
25. Hunyadi J, Farkas B, Bertenyi C, Olah J, Dobozy
A. Keratinocyte grafting: a new means of
transplantation for full-thickness wounds.
J Dermatol Surg Onc. 1988; 14: 75-78.
26. Ronfard V, Rives J-M, Neveux Y, Carsin H, Barrandon
Y. Long-term regeneration of human
epidermis on third degree burns transplanted with autologous cultured
epithelium grown on a fibrin matrix.
Transplantation. 2000; 70:
1588-1598.
27. Fraulin FOG, Bahoric DVM, Harrop AR, Hiruki T, Clarke
HM. Autotransplantation of epithelial
cells in the pig via an aerosol vehicle.
J Burn Care Rehabil 1998; 19: 337-345.
28. Navarro FA, Stoner ML, Lee HB, Park CS, Wood FM,
Orgill DP. Melanocyte repopulation in full-thickness wounds using a cell spray
apparatus. J Burn Care Rehabil. 2001; 22: 41-46.
29. Navarro FA, Stoner ML, Park CS, Huertas JC, Lee HB,
Wood FM, Orgill DP. Sprayed keratinocyte suspensions accelerate epidermal
coverage in a porcine microwound model. J Burn Care Rehabil. 2000;. 21:
513-518.
30. Jiao XY, Kopp J, Tanczos E, Voigt M, Stark GB. Cultured keratinocytes suspended in fibrin
glue to cover full-thickness wounds on athymic nude mice: comparison of two
brands of fibrin glue. Eur J Plast Surg.
1988; 21: 72-76.
31. Chester DL, Balderson DS, Papini RPG. A review of keratinocyte delivery to the
wound bed. J Burn Care Rehabil. 2004;
25: 266-275.
32. Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and
tissue-engineered skin replacements: a review.
Plast Reconstr Surg. 2001; 108: 1713-1726.
33. Walter,RJ, Matsuda,T, Reyes,HM, Walter,JM,
Hanumadass,M. Characterization of
acellular dermal matrices (ADMs) prepared by two different methods. Burns 1998; 24: 104-113.
34. Truong A-H, Kowal-Vern A, Latenser BA, Wiley DE,
Walter RJ. Comparison of dermal
substitutes in wound healing utilizing a nude mouse model. J Burns and Wounds. 2005; 4: 96-107.
35. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular
endothelial growth factor, microvascular hyperpermeability, and
angiogenesis. Am J Pathol. 1995; 146: 1029-1039.
36. Li J, Zhang Y-P, Kirsner RS. Angiogenesis in wound repair: angiogenic
growth factors and the extracellular matrix.
Microsc Res Tech. 2003; 60:
107-114.
37. Lingen MW. Role
of leukocytes and endothelial cells in the development of angiogenesis in inflammation
and wound healing. Arch Pathol Lab Med.
2001; 125: 67-71.
38. Burke JF.
Observations on the development of an artificial skin: presidential
address, 1982 American Burn Association Meeting. J Trauma. 1983; 23: 543-551.
39. Moiemen NS, Staiano JJ, Ojeh, NO, Thway Y, Frame JD.
Reconstructive surgery with a dermal regeneration template: clinical and
histologic study. Plast Reconstr Surg.
2001; 108: 93-103.
40. Stern R, McPherson M, Longaker MT. Histologic study of artificial skin used in
the treatment of full-thickness thermal injury. J Burn Care Rehabil. 1990; 11:
7-13.
41. Sheridan RL, Hegarty M, Tompkins RG, Burke JF. Artificial skin in massive burns-results to
ten years. Eur J Plast Surg. 1994; 17: 91-93.
42. Erdag G, Sheridan RL. Fibroblasts improve performance
of cultured composite skin substitutes on athymic mice. Burns. 2004; 30: 322-328.
FIGURES
Figure 1.
Percent of wound contraction over the 28-day study period for each of the
dermal substitutes. Error bars represent SEM. Data was analyzed by one-way
ANOVA with Tukey’s post tests.
Figure 2. Laminin-stained cross-sections from the wound
center following treatment with KC + FG only, Dermagraft, and Dermalogen on
post-operative day 28 (above) and the corresponding gross appearance of these
wounds at day 28 (below). Extensive vascularization is seen in both the
superficial and deep dermis in all three of these wounds.Sutures mark the
corners of the original wound. Note the
extensive contraction of wounds treated with KC + FG only and Dermalogen®. Dermagraft®-implanted wounds showed poor
epithelialization and poor incorporation into the wound with contraction
limited only as long as the implant was retained. Often part of the Dermagraft® implant
underwent spontaneous dehiscence and was rejected from the wound. Magnification
bar = 100 μm.
Figure 3. Laminin-stained cross-sections from the wound
center following treatment with AlloDerm, ADM, and Integra on post-operative
day 28 (above) and the corresponding gross appearance of these wounds at day 28
(below). Controlled vessel ingrowth is seen in the AlloDerm and ADM groups.
Note the numerous large and small cavities in the Integra® implant. In life, these cavities held the
collagen-chondroitin sulfate colloid that comprises Integra® and this colloid
is still present at this time point. Several large vessels and vessels cut in
tangential or longitudinal section are seen in this specimen. The vessels appear to be growing around the
cavities formed in the Integra implant.
In the gross photographs, sutures mark the corners of the original
wound. Note the limited contraction and
nearly complete epithelialization of these wounds. Magnification bar = 100 μm.
Figure 4. Graph depicting changes in vessel number
(mean±SEM) in the superficial dermis
at the center of the wound over the study period of 28 days. The dashed horizontal line represents the
vascular counts in normal mouse skin (CTL). Note the hypovascularity of the
Integra, AlloDerm, ADM, and Dermalogen groups at day 14 in comparison to CTL,
and the hypervascularity seen with implanted Dermagraft at day 28. Data were
analyzed using one-way ANOVA with Tukey’s post tests.
Figure 5. Graph depicting changes in vessel number
(mean±SEM) in the deep dermis at the
center of the wound over the study period of 28 days. The horizontal dashed line represents the
vascular counts in normal mouse skin (CTL). Data were analyzed using one-way
ANOVA with Tukey’s post tests.
Figure 6. Graph depicting changes in vessel areas
(mean±SEM) in the superficial dermis
at the center of the wound over the study period of 28 days. The dashed horizontal line represents the
vessel size in normal mouse skin (CTL). Note that the vessels in wounds treated
with Integra are larger than CTL at day 14 (p<0.001) and day 28
(p<0.01). Data were analyzed using
one-way ANOVA with Tukey’s post tests.
Figure 7. Graph depicting changes in vessel areas
(mean±SEM) in the deep dermis at the center of the wound over the study period
of 28 days. The dashed horizontal line
represents the vessel size in normal mouse skin (CTL). Note that the vessels in
wounds treated with Integra are larger than CTL at day 14 (p<0.001) and day
28 (p<0.01). Data were analyzed using
one-way ANOVA with Tukey’s post tests.
Figure 8. Graph depicting percentage of original wound area
epithelialized over the study period of 28 days. Error bars represent SEM. At day 28, wounds implanted with AlloDerm,
Integra, or ADM were approximately 40% reepithelialized, whereas wounds
implanted with Dermagraft or Dermalogen were only 10-20% reepithelialized.
Table 1. Table of vessel numbers and sizes (mean±SEM)
in the total dermis (superficial + deep) for each of the groups at day 28.
FYI. The content on this blog is copyright protected by the author.
Feel free to read, copy, and disseminate the studies described here, but
please indicate the origin of the work by citing this blog URL as an
electronic web citation when it is appropriate to recognize and
attribute the work of others.








So much information, really well executed blog. sigma antibody
ReplyDelete