When we were studying the chemotaxis defect known to exist in leukocytes from cancer patients, we looked at formylpeptide receptor dynamics in considerable detail. We examined receptor binding, down-regulation, recovery, and receptor-mediated pinocytosis using rabbit peritoneal PMN, human peripheral PMN, and human peripheral monocytes. The cells were either obtained directly from cancer patients or were from normal subjects. Cells from normal subjects were pretreated with serum that had been obtained from cancer or normal patients.
The full paper and link to the PDF are shown below.
Mechanism of Cancer Chemotaxis Defect
Mechanism of the Cancer-Related Leukocyte Chemotaxis Defect:
Formylpeptide
Receptor Modulation and Pinocytosis
Amelia H. Janeczek, PhD1,3,
Pierson J. Van Alten, PhD1, Hernan M. Reyes, MD2,
and Robert J. Walter, PhD2
1
Department of Anatomy and Cell Biology, University
of Illinois at Chicago, Chicago, IL 60612
2
Department of Surgery, Cook County Hospital, Hektoen Institute for
Medical Research, Chicago, IL 60612
3
Present address: Department of
Biochemistry, School of Medicine, Boston
University, Boston, MA 02118-2394
Address all
correspondence to:
Robert
J. Walter, PhD
Department
of Surgery, Room 905
Hektoen
Institute for Medical Research
625 South Wood Street
Chicago, IL 60612
Telephone: (312) 633-7237/8717 FAX: (312)
738-3102
Running Title:
Mechanism of Cancer Chemotaxis Defect
Keywords:
formylpeptide, receptors, cancer, chemotaxis, endocytosis
SUMMARY
Monocyte
chemotaxis is severely depressed in patients with advanced tumors but the
cellular basis for this chemotactic defect is not known. Pretreatment of normal human leukocytes or
rabbit peritoneal neutrophils (PMN) with serum from cancer (CA) patients
inhibits both monocyte and PMN chemotaxis as compared to leukocytes pretreated
with serum from healthy (CT) blood donors.
Using purified fresh CA patient leukocytes or CA serum-treated normal
leukocytes, formylpeptide receptor binding and modulation were quantified using
radiolabeled formylmethionyl-leucyl-phenylalanine (3H-FMLP). The cell surface binding of 3H-FMLP
at 4°C was significantly reduced in CA serum
pretreated rabbit peritoneal PMN, but not in CA serum pretreated human
peripheral blood PMN or mononuclear leukocytes as compared to CT serum
pretreated cells. However, in purified
mononuclear leukocytes isolated directly from tumor patients, formylpeptide
binding was significantly reduced as compared to those of normal subjects. PMN from tumor patients exhibited no
significant difference in this regard as compared to PMN from control
subjects. The time course and dose
response curves observed during formylpeptide receptor down-regulation were
similar for CT and CA serum pretreated cells.
Subsequent to down-regulation, the recovery of cell surface
formylpeptide binding at 37°C was
similar in its rate and extent in CT and CA serum pretreated cells. However, significant reductions in 3H-FMLP
uptake at 37°C were seen in tumor patient PMN, in CA
serum pretreated rabbit PMN, and in CA serum pretreated human PMN and
mononuclear leukocytes as compared to controls.
Such reductions in initial formylpeptide cell surface binding and in
formylpeptide endocytosis may contribute directly to the cancer-associated
depression of leukocyte chemotaxis.
INTRODUCTION
Monocyte and macrophage chemotaxis is
severely impaired in patients with advanced tumors, but neutrophil (PMN)
locomotion is unaffected (1,2). This
defect in monocyte chemotaxis may have life-threatening consequences since host
leukocytes may be unable to adequately repress bacterial and fungal infections
or tumor growth. Implantation of tumor
cells or injection of tumor sonicates into mice results in reduced accumulation
of macrophages in response to inflammatory stimuli, as well as decreased
resistance to bacterial infection (3,4).
Similarly, treatment of normal leukocytes with serum from patients with
advanced tumors, conditioned media from tumor cell lines, ascites fluid,
plasma, or urine from tumor-bearing mice suppresses monocyte (and often PMN)
polarization and chemotaxis (2,5‑9).
This chemotaxis defect is evident in animals and patients bearing any
of a large number of tumors and is seen in response to several quite different
classes of chemoattractants (for review see 6,10,11). Many studies have shown that monocyte chemotaxis
returns to normal after the surgical removal of tumor or after treatment with
chemotherapy or radiotherapy (12,13).
Taken together, this suggests that tumors may be producing or causing
the host to produce an inhibitor of leukocyte chemotaxis (7,14).
A soluble inhibitor of monocyte
chemotaxis, rendered inactive by treatment with antibody directed against the
retroviral envelope protein p15E, has been found in tumor patient effusions
(15,16) and in serum from patients with head and neck cancer (CA) (17). This inhibitor or its synthetic analogues
may cause decreased monocyte polarization in response to chemoattractant
(8,16), alterations in formylpeptide receptor expression (18,19), suppression
of the respiratory burst (20), and inhibition of protein kinase C-related cell
functions (21). It was hypothesized in
the present study that the chemotactic defect in tumor patient monocytes or in
CA serum-treated leukocytes resulted from alterations in reception or
transduction of the signal for chemotaxis.
To examine this possibility, formylpeptide chemoattractant receptor
binding was examined in leukocytes isolated from tumor patients, in CT or CA
serum pretreated human leukocytes isolated from normal subjects, and in CT or
CA serum pretreated rabbit peritoneal PMN.
Significant
reductions in formylpeptide binding were observed in purified tumor patient
mononuclear leukocytes as well as in CA serum pretreated rabbit peritoneal
PMN. Chemoattractant uptake at 37°C was significantly reduced in CA serum
pretreated rabbit PMN, human PMN, human mononuclear leukocytes, and in tumor
patient PMN. These alterations in
surface binding and internalization of formylpeptide may contribute to the
depression of chemotaxis exhibited by these cells.
Abbreviations used:
BSA, bovine
serum albumin; CA, cancer; CT, control; DMSO, dimethylsulfoxide; EDTA, edetic
acid; FMLP, N-formyl-methionyl-leucyl-phenylalanine; HBS, HEPES buffered salt
solution; HBSS, Hanks' balanced salt solution; HEPES,
N-2-hydroxyethylpiperazine-N-2'-ethanesulfonic acid; MLCK, myosin light chain
kinase; PMN, polymorphonuclear leukocyte.
METHODS AND MATERIALS
Buffers
For
most of the studies described here, HEPES-buffered saline (HBS) containing 140
mM NaCl, 10 mM KCl, 10 mM N-2-hydroxyethylpiperazine N-2-ethanesulfonic
acid (HEPES), 5 mM glucose, and 2 mg/ml bovine serum albumin (BSA), pH 7.4 was
used. For rabbit peritoneal PMN, Hanks'
balanced salt solution (HBSS) containing 10 mM HEPES, 5 mM glucose, 136 mM
NaCl, 5 mM KCl, 373 nM Na2HPO4, 734 nM KH2PO4,
pH 7.2 was used.
Rabbit Peritoneal Neutrophils
Glycogen-elicited
peritoneal PMN were collected from rabbits in heparin-containing tubes on ice,
centrifuged at 500 x g for 10 minutes, and washed in HBSS containing 2 mM
EDTA. Contaminating erythrocytes were
removed by brief hypotonic or ammonium chloride lysis, cells were washed in
HBSS supplemented with 0.2 mM CaCl2 and 1 mM MgSO4, and
kept on ice until use. These cell
preparations consisted of >95% PMN.
Isolation and Purification of Human Leukocytes
Peripheral
venous blood samples were obtained with informed consent from healthy adult
volunteers and from patients admitted to Cook County
Hospital for diagnosis
and treatment of primary head and neck tumors.
Patients included in this study were not receiving chemotherapy,
radiotherapy, or medication at the time of sample collection. Blood was collected by venipuncture in
sterile EDTA-containing tubes and leukocytes isolated by a modification of the
method of Boyum (22). Erythrocytes were
gravity-sedimented at room temperature by the addition of pyrogen-free dextran
(200 kD) to a final concentration of 1.25%.
The leukocyte-rich plasma was diluted with an equal volume of HBS with
EDTA, layered onto a cushion of Lymphocyte Separation Medium (Organon Teknika
Corp, Durham, NC) and centrifuged at 500 x g for 5 min at
room temperature. Mononuclear leukocytes
at the interface of the discontinuous gradient were collected, diluted with HBS
containing EDTA and 2.5% dextran, and centrifuged at 250 x g for 5 min at room
temperature. The platelet-rich
supernatant was removed and this washing procedure repeated twice. The PMN pellet was resuspended in HBS with
EDTA and centrifuged at 5000 x g in a microcentrifuge for 2 seconds at room
temperature. Contaminating erythrocytes
were removed by brief hypotonic lysis and the leukocytes washed three times in
HBS with EDTA. Mononuclear leukocytes
and PMN were finally resuspended in HBS containing divalent cations and kept on
ice until use.
Serum Pretreatment
Blood
samples from healthy adult donors and patients with head and neck tumors were
collected by venipuncture and allowed to clot overnight at 4°C.
Serum was collected by centrifugation and stored frozen in aliquots at
-80°C until use. Purified human peripheral blood PMN or
mononuclear leukocytes were pretreated by incubation with 10% human serum in
HBS with EDTA at 37°C for 30 min
in siliconized glass test tubes. The
cells were then centrifuged at 600 x g for 3 min at room temperature and
resuspended in HBS with divalent cations at 4°C.
Chemotaxis Assays
N-formyl-methionyl-leucyl-phenylalanine
(FMLP; Peninsula Laboratories, Belmont, CA) in concentrations ranging from 0.1
to 100 nM was placed in the bottom wells of a 48 well chemotaxis chamber (Neuroprobe,
Cabin John, MD) and covered with a 5 μm pore size polyvinylpyrrolidone-free
filter (Nucleopore Corp, Pleasanton, CA) as described previously (10,19). Briefly, control and CA serum pretreated
human PMN or purified mononuclear leukocytes (2-7 x 104 cells) or
rabbit peritoneal PMN (2 X 104 cells) were loaded into the upper
wells and the chambers were incubated at 37°C for 2 hours. The
filters were removed, fixed in methanol, stained, rinsed, dried, and mounted on
glass slides using Permount. Assays were
quantitated by counting the number of cells in 5 contiguous 40X microscope
fields (23).
3H-FMLP Binding and
Uptake Studies
Mononuclear
leukocytes (350,000/ 100 μl) or PMN (1 X 106/ 100 μl) were allowed
to adhere to acid-cleaned 12 mm diameter round glass coverslips in a humidified
chamber at 37°C for 10 minutes. Control or CA serum was added to each
coverslip to a final concentration of 10%, and incubation continued for 30
minutes at 37°C.
Serum was removed by washing the coverslips in HBSS and these
preparations studied as described below.
Cell viability remained greater than 90% throughout these experiments. No cell loss was detected by visual
inspection or cell counts of coverslips, and buffer pH was maintained between
7.4 and 7.6.
Baseline FMLP Receptor Binding at 4°C
Adherent,
serum pretreated cells on coverslips were rinsed thoroughly in cold HBSS, then
incubated in 75 μl of 20 nM 3H-FMLP (58 Ci/mmole; New England
Nuclear, Boston, MA) for 60 min at 4°C. The coverslips
were washed briefly in 2 changes of fresh HBSS, immersed in scintillation
cocktail (Biofluor; New England Nuclear, Boston,
MA), and cell-associated radioactivity
determined by scintillation counting (Tm Analytic Inc., Elk Grove Village, IL).
Formylpeptide Receptor Down-Regulation
To
establish a concentration curve for FMLP-induced receptor down- regulation,
coverslip-adherent rabbit PMN were pretreated with either CT or CA serum,
incubated with varying concentrations of unlabeled FMLP (0.1 - 20 nM) for 20
min at 37°C, rinsed thoroughly in fresh cold
buffer, and then exposed to 20 nM 3H-FMLP for 60 min at 4°C.
Baseline cell-associated 3H-FMLP levels were determined in
coverslip-adherent cells that had not previously been exposed to unlabeled
FMLP.
Receptor
down-regulation over a 20 min time course was studied for cells exposed to 5 nM
unlabeled FMLP at 37°C. Subsequently, coverslips were rinsed in cold
HBSS, and cell surface formylpeptide receptor expression was assessed using 3H-FMLP
as described above.
Formylpeptide Receptor Reexpression on
the Cell Surface
Adherent,
serum pretreated cells were incubated with 5 nM unlabeled FMLP at 37°C for 20 min to down-regulate
formylpeptide receptors, rinsed well with 4 changes of fresh HBSS for 10 min on
ice to permit dissociation of surface-bound FMLP, and then further incubated in
HBSS for 0 to 60 min at 37°C to allow
receptor reexpression on the cell surface.
After these manipulations, the coverslips were rinsed well in cold HBSS,
and cell surface formylpeptide receptor expression assessed as described above.
3H-FMLP Uptake
Control
and CA serum pretreated adherent cells were incubated with 75 μl of 20 nM 3H-FMLP
at 37°C in a humidified chamber for times
ranging from 0 to 60 minutes. At each
time point, coverslips were washed in cold HBSS, immersed in BioFluor, and
cell-associated radioactivity determined by scintillation counting.
Statistical Evaluation of Data
Samples
were run in triplicate and means of these triplicate groups were compared using
Student's paired or unpaired t-tests.
Probability values less than 0.05 were considered significant (24).
RESULTS
Chemotaxis is Reduced in CA Patient
Leukocytes and after Pretreatment of Normal Leukocytes with CA Serum
Mononuclear
leukocytes from CA patients and PMN or mononuclear leukocytes pretreated with
serum showed reduced chemotaxis in response to FMLP as compared to normal
leukocytes or to cells similarly pretreated with CT serum. Since this phenomenon has been described in
detail elsewhere (6,7,10,11), statistics descriptive of the samples used here
will only be mentioned. Leukocytes
isolated from 6 different CA patients, 17 different CA serum samples, and 8
different CT serum samples were employed.
On the average chemotaxis was reduced in CA mononuclear leukocytes (64%,
6 trials), in CA serum pretreated rabbit peritoneal PMN (40%, 6 trials), in CA
serum pretreated human PMN (24%, 24 trials), and in CA serum pretreated human
mononuclear leukocytes (60%, 34 trials).
Relative to that seen with CT serum, CA serum alone exhibited no
significant chemotactic activity for either PMN or mononuclear leukocytes.
Initial 3H-Formylpeptide Binding
on the Cell Surface at 4°C
Cancer
serum pretreated rabbit PMN bound an average of 10% less 3H-FMLP
than cells similarly treated with CT serum (p<0.01; paired t-test; Figure
1A). When human PMN and mononuclear
leukocytes from normal controls were studied, no differences in 3H-FMLP
binding in CT as compared to CA serum pretreated cells were observed. In contrast, mononuclear leukocytes isolated
from head and neck CA patients bound 42% less 3H-FMLP (p<0.05;
Figure 1B), whereas CA patient PMN showed no significant differences in 3H-FMLP
binding.
The specificity of 3H-FMLP
binding was tested by exposing coverslip-adherent PMN or mononuclear leukocytes
to 20 nM 3H-FMLP in the presence of excess (20 μM) unlabeled
FMLP. Non-specific binding ranged from
1.6% to 5.3% of total 3H-FMLP binding in the experiments reported
here and has been subtracted from the total binding to give receptor-specific
binding. Calculations of formylpeptide
receptor numbers indicated approximately 43,000 as compared to 36,000 receptors
per cell for rabbit PMN pretreated with CT as compared with CA serum. Similar numbers of formylpeptide receptors on
rabbit PMN have been reported elsewhere (25,26).
Formylpeptide Receptor Down-Regulation is
not Altered by CA Serum
1. Formylpeptide Concentration
Curve
The
response of CT or CA serum pretreated rabbit PMN to challenge with unlabeled
formylpeptide at 37°C was
assessed over a range of FMLP concentrations (Figure 2). In the absence of FMLP (i.e., 0 nM in Figure
2), there was no significant difference in 3H-FMLP binding between
CT and CA serum pretreated PMN. Note
that the preparation procedure for cells at this data point differed from that
described for initial formylpeptide binding (previous section). After serum treatment, an additional 20 min
buffer incubation at 37°C and 10 min
buffer incubation at 4°C was
employed. This extended incubation time
after serum treatment may have affected the FMLP binding on the cell surface
such that a significant difference (as noted in previous section) no longer
existed between the CT and CA serum pretreated groups. The amount of 3H-FMLP binding
varied inversely with the concentration of chemoattractant used during
preincubation. With 5 nM unlabeled FMLP,
3H-FMLP counts were 27% and 25% of baseline for CT and CA sera
pretreated cells, respectively.
Treatment with higher concentrations of unlabeled chemoattractant did
not reduce 3H-FMLP binding beyond the level seen with 5 nM FMLP. At each concentration of unlabeled FMLP used,
3H-FMLP binding was similar in the CT and CA serum pretreated
groups. Since a substantial reduction of
cell-associated 3H-FMLP was obtained with 5 nM unlabeled FMLP,
further down-regulation studies were performed using this concentration.
2.
Down-Regulation Time Course
Figure
3 shows that CA serum pretreated rabbit PMN not exposed to unlabeled FMLP
(i.e., 0 time) showed 14% less 3H-FMLP binding than the corresponding
CT serum pretreated group (p<0.02).
With increasing incubation time in unlabeled chemoattractant, cell
surface 3H-FMLP binding decreased.
Clearance of receptors was rapid, with more than 50% of the original
receptor-mediated binding lost after 2 min of exposure to unlabeled FMLP. The majority of receptors (78% in CT serum
pretreated cells and 75% in CA serum pretreated cells) were cleared within 5
min after initial exposure to unlabeled FMLP.
After 20 min of incubation in unlabeled FMLP, only 9% of original cell
surface binding remained in both groups.
No additional significant differences were noted between CT and CA serum
pretreated groups during the 20 min down-regulation time course.
Formylpeptide Receptor Reexpression is
not Altered by CA Serum
Coverslip-adherent
rabbit PMN pretreated with CT or CA serum were first exposed to 5 nM unlabeled
FMLP for 20 min, rinsed, and then further incubated in buffer for up to 60 min
at 37°C to allow receptor reexpression on the
cell surface. Cells exposed to buffer
alone at 37°C instead of 5 nM unlabeled FMLP showed a
uniform, high level of 3H-FMLP binding throughout the 60 min observation
period (i.e., 100% of receptors present on cell surface). As seen in Figure 4, PMN exposed to 5 nM
unlabeled FMLP for 20 min at 37°C showed greatly decreased cell surface FMLP binding (30%
and 32% of initial binding for CT or CA serum pretreated cells, respectively). Upon further incubation in fresh buffer at 37°C, increasing amounts of 3H-FMLP
binding were observed. However, no
significant differences in the rate or extent of formylpeptide receptor
reexpression were seen in CT as compared to CA serum pretreated PMN.
Uptake of 3H-Formylpeptide at
37°C
is Diminished in CA Leukocytes
When
leukocytes were incubated with 3H-FMLP at 37°C, the amount of cell-associated peptide
increased with time. For rabbit PMN, a
rapid increase in 3H-FMLP accumulation was evident within 2 minutes
(Figure 5), but uptake by CT or CA serum pretreated cells did not differ
significantly at this time point.
Thereafter, the amount of cell-associated 3H-FMLP gradually
increased reaching a peak at 20 minutes, after which time it remained
constant. Cancer serum pretreated cells
showed significantly reduced uptake of 3H-FMLP at 10, 20, and 40 min
compared to CT serum pretreated cells (p<0.015).
Serum
pretreated human PMN and mononuclear leukocytes exhibited a slower accumulation
of 3H-FMLP than did rabbit PMN.
During the initial 10 min of exposure to 20 nM 3H-FMLP at 37°C, uptake by CA serum pretreated human
leukocytes was not significantly different from that of CT serum pretreated
human leukocytes (Figure 6). Upon
further incubation, 3H-FMLP continued to accumulate such that the
uptake of 3H-FMLP in the CA serum pretreated leukocytes was 20-45%
less than that of the CT serum pretreated leukocytes (10 min, p<0.05; 40
min, p<0.01; 60 min, p<0.04).
Neutrophils isolated from tumor patients and CT subjects (Figure 7)
exhibited patterns of 3H-FMLP uptake similar to those observed in
serum pretreated leukocytes. In these
samples, uptake of 3H-FMLP was significantly reduced in CA patient
PMN at 20, 40 and 60 min (20 min, p<0.001; 40 min, p<0.001; 60 min,
p<0.002) as compared to that in CT patient PMN.
DISCUSSION
In
the present study, CA patient mononuclear leukocytes as well as rabbit
peritoneal PMN, normal human mononuclear leukocytes, and normal human PMN
pretreated with 10% CA serum showed consistent reductions in
formylpeptide-mediated chemotaxis when compared to leukocytes pretreated with
CT serum. Significant reductions in
formylpeptide binding were observed in tumor patient mononuclear leukocytes as
well as in CA serum pretreated rabbit peritoneal PMN. However, formylpeptide receptor modulation,
i.e., down-regulation from and receptor reexpression onto the cell surface, in
CA serum pretreated leukocytes was similar to that seen in CT serum pretreated
cells. Nonetheless, significant
reductions in 3H-FMLP uptake at 37°C were seen in CA serum pretreated rabbit PMN, human PMN,
human mononuclear leukocytes as compared to CT serum pretreated leukocytes and
also in tumor patient PMN as compared to PMN from normal subjects.
Human
CA serum inhibits chemotaxis of normal human PMN and monocytes (7) as well as
normal guinea pig PMN (27). Monocytes
isolated from CA patient blood display a similar inhibition of chemotaxis, but
chemotaxis of PMN from CA patients is not impaired (1,23,28). The reason for this disparity is not apparent
but similar findings were obtained here.
In addition, initial formylpeptide binding was reduced for rabbit PMN
pretreated with CA serum as compared to CT serum pretreated cells but no
significant differences were noted between CT and CA serum pretreated human
leukocytes. For leukocytes isolated
directly from CA patient blood samples, formylpeptide binding was not
significantly different for PMN but was significantly reduced (40%) for
mononuclear leukocytes as compared to that of leukocytes from normal control
subjects. Similarly, Oostendorp et al.
(18) reported decreased formylpeptide binding by human monocytes and PMN in
the presence of the p15E-related peptide, LDLLFL. If initial formylpeptide binding is reduced,
as it is for CA mononuclear leukocytes, then decreased chemotactic
responsiveness may be the direct result.
Although CA serum pretreatment resulted in decreased chemotaxis in
human PMN and mononuclear leukocytes, formylpeptide binding was not altered.
To
further evaluate the role of formylpeptide receptors in the cancer-associated
chemotactic defect, cell surface receptor down-regulation and reexpression were
examined. The results of these studies
were consistent with previous reports in which rabbit (29,30) or human (31,32)
PMN were used to show that receptor-ligand complexes are removed from the cell surface
by internalization resulting in a net decrease in cell surface formylpeptide
receptor expression (32‑35). There were
no significant differences between CT and CA serum pretreated PMN with regard
to their formylpeptide receptor complement at any time (except at 0 time) or at
any FMLP concentration employed here.
Thus, it seems that the decrease in chemotactic responsiveness in the CA
serum pretreated cells is not due to alterations in the receptor
down-regulation response or altered clearance of occupied cell surface
formylpeptide receptors as seen under these conditions.
Control and CA serum pretreated rabbit PMN
were initially exposed to unlabeled FMLP for 20 min to induce receptor
down-regulation, rinsed in fresh buffer, further incubated at 37°C for varying times to permit receptor
reexpression on the cell surface, and the binding capacity of the cells
determined using 3H-FMLP.
With increasing incubation time at 37°C, a gradual increase in 3H-FMLP binding occurred
but complete receptor reexpression was not observed. Several possible technical reasons for this
incomplete recovery were explored including possible cell loss, shifts in
buffer pH, and nutritional deficiencies of the incubation media, but none of
these variables could account for the finding that formylpeptide receptor
reexpression never exceeded 85% of the initial cell surface binding. Nonetheless, no differences were seen in
formylpeptide receptor reexpression in CT and CA serum pretreated cells. Thus, the chemotactic defect in the CA serum
pretreated group does not seem to result from altered or insufficient
formylpeptide receptor reexpression.
A
significant decrease in 3H-FMLP uptake was seen in rabbit PMN and in
normal human PMN and mononuclear leukocytes when they were pretreated with CA
serum. A similar decrease was observed
in PMN from CA patients. This decrease
was not seen at early time points, but became apparent after 20 minutes of
exposure to labeled FMLP. Others have
shown that Fc receptor-mediated phagocytosis of radiolabeled immune complexes
was suppressed in human PMN and monocytes treated with fractions prepared from
CA patient serum (7) and that phagocytosis was reduced in tumor patient
monocytes (36). Further, Naik et al.
(37) found that fluid pinocytosis in PMN from patients with chronic myeloid
leukemia was significantly reduced as compared to that of normal PMN. Although phagocytosis, receptor-mediated
endocytosis, and fluid-phase pinocytosis are distinctly different processes
(38), it seems that CA serum may have an inhibitory effect on all three forms
of endocytosis. Accumulation of soluble 3H-FMLP
at 37°C involves two concurrent cellular
processes, receptor-mediated endocytosis (saturable uptake) and fluid-phase
pinocytosis (non-saturable uptake). The
method employed here did not permit the contributions of each of these
processes to be distinguished, but previous studies employing identical
conditions have concluded that about 80% of formylpeptide uptake occurs by a
receptor-mediated mechanism (29,39).
Moreover, it is evident from Figure 5 that uptake by rabbit PMN was
saturable and thus primarily receptor-mediated.
Finally, preliminary experiments using 14C-polyethylene
glycol, a marker for fluid phase pinocytosis, have shown no differences in
uptake between CT and CA serum pretreated leukocytes after formylpeptide
stimulation (unpublished data). There is, however, an apparent contradiction
between this finding (inhibition of receptor-mediated uptake) and the results
of experiments on formylpeptide receptor down-regulation and reexpression which
were not affected by CA serum pretreatment.
Although the latter are not altered in CA leukocytes under the
conditions described, it is possible that the effects of the CA serum may have
dissipated during the lengthy incubations (60-180 min after completion of serum
pretreatment) required to perform these experiments. Inhibitory effects of serum may remain
evident, however, in the former experiments since they required much shorter
incubations (5-60 min) after serum treatment.
Further studies will be required to evaluate this possibility.
In
general, formylpeptide receptor down-regulation and receptor reexpression did
not appear to be affected by pretreatment with cancer serum. However, significant reductions in
formylpeptide binding were observed in tumor patient mononuclear leukocytes and
in cancer serum pretreated rabbit peritoneal PMN. Reductions in chemoattractant uptake at 37°C were seen in cancer serum pretreated
rabbit PMN, human PMN, human mononuclear leukocytes and in tumor patient
PMN. These alterations in surface
binding and internalization of formylpeptide may contribute to the depression
of chemotaxis exhibited by these cells.
Further characterization of CA leukocytes and the effects of CA serum on
leukocyte chemotaxis and fluid phase pinocytosis are in progress.
REFERENCES
1.
Rubin RH, Cosimi AB,
Goetzl EJ: Defective human mononuclear leukocyte chemotaxis as an index of host
resistance to malignant melanoma. Clin Immunol Immunopathol 1976, 6:376‑388.
2. Snyderman R, Pike MC,
Blaylock BL, Weinstein P: Effects of neoplasms on inflammation: depression of
macrophage accumulation after tumor implantation. J Immunol 1976, 116:585‑589.
3. Snyderman R, Pike MC: An
inhibitor of macrophage chemotaxis produced by neoplasms. Science 1976, 192:370‑372.
4. Pike MC, Snyderman R:
Depression of macrophage function by a factor produced by neoplasms a mechanism
for abrogation of immune surveillance. J Immunol 1976, 117:1243‑1249.
5. Snyderman R, Cianciolo GJ:
Further studies of a macrophage chemotaxis inhibitor (MCI) produced by
neoplasms: murine tumors free of lactic dehydrogenase virus produce MCI. J
Reticuloendothel Soc 1979, 26:453‑458.
6. Balm FJM, von Blomberg‑van
deFlier BME, Drexhage HA, de Haan‑Meulman M, Snow GB: Mononuclear phagocyte
function in head and neck cancer: depression of murine macrophage accumulation
by low molecular weight factors derived from head and neck carcinomas.
Laryngoscope 1984, 94:223‑227.
7. Maderazo EG, Anton TF,
Ward PA: Serum‑associated inhibition of leukotaxis in humans with cancer. Clin
Immunol Immunopathol 1978, 9:166‑176.
8. Cianciolo GJ, Snyderman R:
Characterization of an inhibitor of monocyte function in effusions of cancer
patients. Lymphokines and Thymic Hormones: Their Potential Utilization in
Cancer Therapeutics. Edited by Goldstein AL,
Chirigos MA. New York,
Raven Press, 1981, pp. 205‑213.
9. Ji‑Ming W, Cianciolo GJ,
Snyderman R, Mantovani A: Coexistence of a chemotactic factor and a retroviral
P15E‑like related chemotaxis inhibitor in human tumor cell culture
supernatants. J Immunol 1986, 137:2726‑2732.
10. Walter
RJ, Danielson JA, Van Alten PJ, Powell WJ: Defects in monocyte chemotaxis in
patients with neoplastic disease. J Surg Res 1986, 41:215‑224.
11. Cianciolo
GJ: Anti‑inflammatory proteins associated with human and murine neoplasms.
Biochim Biophys Acta 1986, 865:69‑82.
12. Snyderman
R, Seigler HF, Meadows L: Abnormalities in monocyte chemotaxis in patients with
melanoma: Effects of immunotherapy and tumor removal. J Natl Cancer Inst 1977,
58:37‑41.
13. Snyderman
R, Meadows L, Holder W, Wells S: Abnormal monocyte chemotaxis in patients with
breast cancer: Evidence for a tumor mediated effect. J Natl Cancer Inst 1978,
60:737‑740.
14. Cianciolo
GJ, Matthews TJ, Bolognesi DP, Snyderman R: Macrophage accumulation in mice is
inhibited by low molecular weight products from murine leukemia viruses. J
Immunol 1980, 124:2900‑2905.
15. Cianciolo
GJ, Hunter J, Silva J, Haskill TS, Snyderman R: Inhibitors of monocyte response
to chemotaxins are present in human cancerous effusions and react with
monoclonal antibodies to the P15(E) structural protein of retroviruses. J Clin
Invest 1981, 68:831‑844.
16. Tan
IB, Drexhage HA, Scheper RJ, et al: Defective monocyte chemotaxis in patients
with head and neck cancer. Arch Otolaryngol HN Surg 1986, 112:541‑544.
17. Tan
IB, Balm AJM, Snow GB, Drexhage HA: Immunosuppressive retroviral‑related
factors in sera of patients with head and neck cancer. Eur Arch
Otorhinolaryngol 1990, 247:387‑390.
18. Oostendorp
RAJ, Knol EF, Verhoeven AJ, Scheper RJ: An immunosuppressive retrovirus‑derived
hexapeptide interferes with intracellular signaling in monocytes and
granulocytes through N‑formylpeptide receptors. J Immunol 1992, 149:1010‑1015.
19. Walter
RJ, Danielson JR: Characterization of formylpeptide chemoattractant binding on
neutrophils and monocytes from patients with head and neck cancer. J Natl
Cancer Inst 1987, 78:61‑69.
20. Harrell
RA, Cianciolo GJ, Copeland TD, Oroszlan S, Snyderman R: Suppression of the
respiratory burst of human monocytes by a synthetic peptide homologous to
envelope proteins of human and animal retroviruses. J Immunol 1986, 136:3517‑3520.
21. Gottlieb
RA, Kleinerman ES, O'Brian CA, Tsujimoto S, Cianciolo GJ, Lennarz WJ:
Inhibition of protein kinase C by a peptide conjugate homologous to a domain of
the retroviral protein p15E. J Immunol 1990, 145:2566‑2570.
22. Boyum
A: Isolation of mononuclear cells and granulocytes from human blood. Scand J
Clin Lab Invest 1968, 21:77‑89.
23. Walter
RJ, Danielson JR: Defective monocyte chemotaxis in patients with epidermoid
tumors of the head and neck. Arch Otolar 1985, 111:538‑540.
24. Sokal
RR, Rohlf FJ: Biometry. New York, W.H. Freeman and Co., 1981, pp. 62‑97.
25. Mackin
WM, Huang C‑K, Becker EL: The formylpeptide chemotactic receptor on rabbit
peritoneal neutrophils. J Immunol 1982, 129:1608‑1611.
26. Aswanikumar
S, Corcoran B, Schiffman E, et al: Demonstration of a receptor on rabbit
neutrophils for chemotactic peptides. Biochem Biophys Res Commun 1977, 74:810‑817.
27. Miyahara
T, Torisu M: Serum inhibitory factor for guinea pig macrophage and neutrophil
chemotaxis found in cancer patients. Gann 1981,72:854‑861.
28. Snyderman R, Pike MC: Defective macrophage
migration produced by neoplasms: identification of an inhibitor of macrophage
chemotaxis. The Macrophage in Neoplasia. Edited by Fink MA. New York, Academic Press, 1976, pp. 49‑65.
29. Zigmond
SH, Sullivan SJ, Lauffenburger DA: Kinetic analysis of chemotactic peptide
receptor modulation. J Cell Biol 1982, 92:34‑43.
30. Janeczek
AH, Marasco WA, Van Alten PJ, Walter RJ:
Autoradiographic analysis of formylpeptide chemoattractant binding, uptake and
intracellular processing by neutrophils. J Cell Sci 1989, 94:155‑168.
31. Sklar
LA, Finney DA, Oades ZG, Jesaitis AJ, Painter RG, Cochrane CG: The dynamics of
ligand‑receptor interactions. Real‑time analysis of dissociation, and
internalization of an N‑formylpeptide and its receptors on the human
neutrophil. J Biol Chem 1984, 259:5661‑5669.
32. Niedel
JE, Kahane I, Cuatrecasas P: Receptor‑mediated internalization of fluorescent
chemotactic peptide by human neutrophils. Science 1979, 205:1412‑1414.
33. Sullivan
SJ, Zigmond SH: Chemotactic peptide receptor modulation in polymorphonuclear
leukocytes. J Cell Biol 1980, 85:703‑711.
34. Jesaitis
AJ, Naemura JR, Painter RG, Schmitt M, Sklar LA, Cochrane CG: The fate of the N‑formyl‑chemotactic
peptide receptor in stimulated human granulocytes: Subcellular fractionation
studies. J Cell Biochem 1982, 20:177‑191.
35. Sklar
LA, Jesaitis AJ, Painter RG, Cochrane CG: Ligand/receptor internalization: a
spectroscopic analysis and a comparison of ligand binding, cellular response,
and internalization by human neutrophils. J Cell Biochem 1982,
20:193‑202.
36. Kunz
BME, Kunz RM, Albert ED: Phagocytosis of monocytes in cancer patients. Zeit
Krebsforsch 1978, 91:11‑17.
37. Naik NR, Advani SH, Bhisey AN: Fluid
pinocytosis and esterase‑oxidase in chronic myeloid leukemic granulocytes are
differentially stimulated by chemotactic peptide. Leuk Res 1992,
16:395‑401.
38. Silverstein
SC, Steinman RM, Cohn ZA: Endocytosis. Ann Rev Biochem 1977, 46:669‑722.
39.
Daukas G, Zigmond SH: Inhibition of
receptor‑mediated but not fluid‑phase endocytosis in polymorphonuclear
leukocytes. J Cell Biol 1985, 101:1673‑1679.
FIGURES
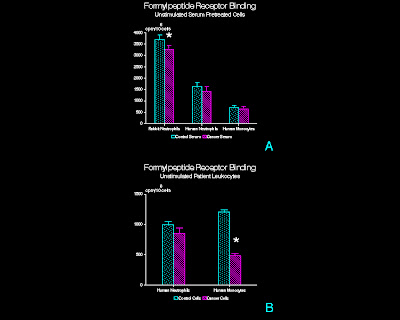
Figure 1
Binding of 3H-FMLP
to adherent, unstimulated leukocytes exposed to 3H-FMLP for 60 min
at 4°C.
(A) Serum pretreated normal leukocytes. Adherent rabbit peritoneal PMN, human
peripheral blood PMN, and human peripheral blood mononuclear leukocytes were
pretreated with CT or CA serum prior to exposure to 3H-FMLP. (n=15, rabbit PMN; n=20, human PMN; n=12,
human mononuclear leukocytes; mean + SD)
* p<0.01 (B)
Patient peripheral blood leukocytes.
PMN and mononuclear leukocytes isolated from venous blood samples
obtained from tumor patients or control subjects were exposed to 3H-FMLP
at 4°C.
(n=3; mean + SD) * p<0.05
Figure 2
Down-regulation
of formylpeptide receptors in the presence of varying concentrations of
unlabeled FMLP by adherent rabbit peritoneal PMN pretreated with CT or CA
serum. Shown is a representative
experiment (n=4; mean + SD).
Figure 3
Time course of
formylpeptide receptor down-regulation for adherent rabbit peritoneal PMN
pretreated with CT or CA serum and incubated with 10 nM unlabeled FMLP. Shown is a representative experiment (n=4;
mean + SD).
Figure 4
Formylpeptide
receptor recovery after down-regulation for adherent rabbit peritoneal PMN
pretreated with CT or CA serum, exposed to 5 nM unlabeled FMLP, rinsed, and
further incubated in buffer at 37°C. Shown is the
percent of cell surface receptors expressed as compared to cell samples not
exposed to unlabeled FMLP (i.e., 100% receptor expression) (n=3; mean + SD).
Figure 5
Uptake of 3H-FMLP
(20 nM) at 37°C by adherent rabbit PMN pretreated with
CT or CA serum. Uptake of 3H-FMLP
in the CA serum pretreated group is significantly reduced at 10 min
(p<0.003), 20 min (p<0.001) and 40 min (p<0.015). Shown is a representative experiment (n=8).
Figure 6
Uptake of 3H-FMLP
at 37°C in the presence of 20 nM 3H-FMLP
by adherent normal human leukocytes pretreated with CT or CA serum. (A)
Uptake in the CA serum pretreated cells is significantly reduced at 40
min (p<0.01) and 60 min (p<0.04).
Shown is a representative experiment (n=6; mean + SD). (B)
Uptake in CA serum pretreated mononuclear leukocytes is significantly
reduced at 40 min (p<0.01) in the CA serum pretreated cells. Shown is a representative experiment (n=5;
mean + SD).
Figure 7
Uptake of 3H-FMLP
at 37°C in the presence of 20 mM 3H-FMLP
by adherent PMN isolated from control subjects and tumor patients. Uptake in the CA patient PMN is significantly
reduced at 20 min (p<0.001), 40 min (p<0.001) and 60 min
(p<0.002). Shown is a representative
experiment (n=3; mean + SD).
FYI. The content on this blog is copyright protected by the author. Feel free to read, copy, and disseminate the studies described here. When it is appropriate to recognize and attribute the work of others, please indicate the origin of the work by citing this blog URL as an electronic web citation .






No comments:
Post a Comment